MedTech News
.................... by Andrew Celentano

Tyber Medical Anatomical Plating System Cleared For Canada
BETHLEHEM, Pa., Feb. 6, 2024 /PRNewswire/ — Tyber Medical LLC, a leading orthopedic device manufacturer providing private label orthopedic implants for the trauma, extremity, and spine markets, received clearance for the anatomical plating system in Canada. The comprehensive portfolio previously received FDA 510(k) in the US and has now been cleared through Health Canada.

FDA Clears Groundbreaking Corplex P™: The First Human Umbilical Cord-Derived Medical Device
ROSWELL, Ga., Feb. 6, 2024 /PRNewswire/ — StimLabs® announces FDA 510(k) clearance of Corplex P, the pioneering human umbilical cord-derived medical device. This first-of-its-kind clearance marks a significant milestone for the wound care industry and highlights StimLabs’ position at its forefront.

FDA expands approval for Boston Scientific spinal cord stim to include chronic back pain without prior back surgery
Boston Scientific (NYSE: BSX)+
announced today that the FDA approved an expanded indication for its WaveWriter spinal cord stimulation (SCS) systems.
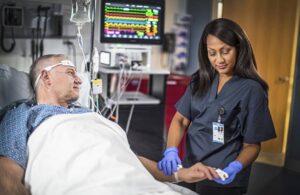
Philips wins FDA nod for latest IntelliVue patient monitor software including Sounds alarm package
Philips (NYSE: PHG)+
announced today that it received FDA 510(k) clearance for its latest IntelliVue patient monitor software.
Aurora Spine’s Second Patent Related to DEXA Technology™ Patient-Matched Implant Technology Issued by the United States Patent Office
CARLSBAD, Calif., Feb. 05, 2024 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced the issuance of its second United States Patent No: 11,850,162 entitled “Body Density Scan Result-Matched Orthopedic Implants and Methods of Use” for The World’s First DEXA Technology™ Patient-Matched Implant Technology.
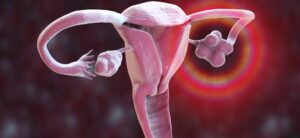
New diagnostic method approved for PCOS
Roche Diagnostics has announced CE mark approval for a claim extension to the Elecsys Anti-Müllerian Hormone (AMH) Plus immunoassay. The claim extension means that the blood test, already available on the NHS, can now be used by physicians to help diagnose women suspected of having polycystic ovary syndrome (PCOS) as an alternative to a transvaginal ultrasound.

FDA clears new cervical cytology AI tech from Hologic
Hologic (Nasdaq: HOLX)+
announced that the FDA granted clearance for its Genius digital diagnostics system with the Genius cervical AI algorithm.
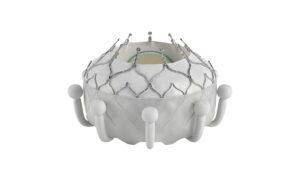
Edwards receives FDA approval for first transcatheter tricuspid valve replacement treatment
The Evoque valve will serve patients with few treatment options, wrote RBC analyst Shagun Singh.
