MedTech News
.................... by Andrew Celentano
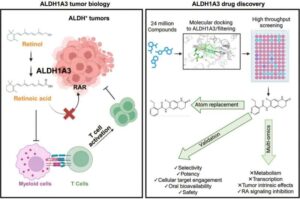
Immune sabotage: How a Vitamin A byproduct compromises the body’s normal anti-cancer response
Scientists at the Princeton University Branch of the Ludwig Institute for Cancer Research have identified novel mechanisms by which a metabolic derivative of vitamin A—all-trans retinoic acid—compromises both the body’s normal anti-cancer immune response and, in a different context, the efficacy of a promising type of cancer vaccine.
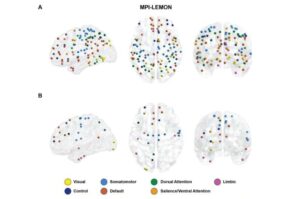
Mathematics uncovers shifting brain connectivity in autism and aging
Researchers from the Max Planck Institute for Mathematics in the Sciences Leipzig, Germany, the Institute of Mathematical Sciences in Chennai, India, and colleagues demonstrate how mathematical techniques from topological data analysis (TDA) can provide a new, multiscale perspective on brain connectivity.
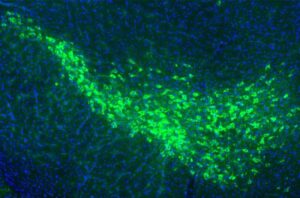
Proteins that spread Parkinson’s pathology in the brain identified
Two proteins found on the surface of motor neurons in the brain may be essential in the progression of Parkinson’s disease, according to new Yale School of Medicine (YSM) research.
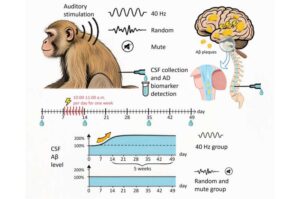
Successful 40-Hz auditory stimulation in aged monkeys suggests potential for noninvasive Alzheimer’s therapy
A research team from the Kunming Institute of Zoology (KIZ) of the Chinese Academy of Sciences has demonstrated for the first time in non-human primates that auditory stimulation at 40 Hz significantly elevates β-amyloid levels in the cerebrospinal fluid (CSF) of aged rhesus monkeys, with this effect persisting for over five weeks.
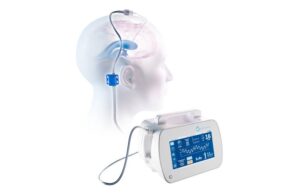
BrainSpace wins FDA clearance for automated brain fluid management device
BrainSpace announced that the FDA granted 510(k) clearance for its Intellidrop automated brain fluid management system.

Ceribell gets FDA breakthrough nod for stroke detection, monitoring solution
Ceribell (Nasdaq:CBLL) announced today that the FDA granted breakthrough device designation for its large vessel occlusion (LVO) stroke detection monitor.
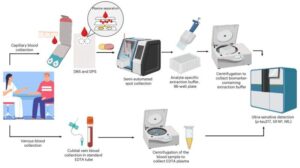
Remote Alzheimer’s testing: At-home blood tests can accurately detect key biomarkers
A new international study has demonstrated that Alzheimer’s disease biomarkers can be accurately detected using simple finger-prick blood samples that can be collected at home and mailed to laboratories without refrigeration or prior processing.
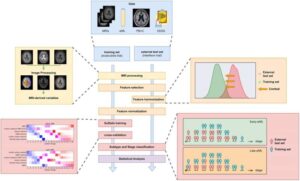
AI uncovers two distinct multiple sclerosis types
Artificial intelligence (AI), using a simple blood test combined with standard brain images has, for the first time, been able to identify two biologically distinct types of multiple sclerosis (MS).
