MedTech News
.................... by Andrew Celentano

FDA clears HeartBeam’s at-home arrhythmia assessment tool
HeartBeam’s ECG software duplicates the 12-lead ECG approach undertaken in healthcare settings with electrodes to evaluate heart arrhythmias.

Roche gains CE Mark for bacterial vaginosis/candida vaginitis assay
The assay is currently available in nations that recognise the CE Mark.
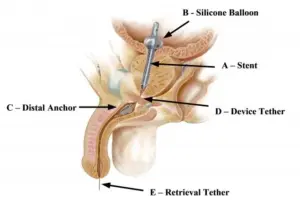
FDA Approves ProVee System, New Generation of Prostatic Urethral Stents
The ProVee System is a next-generation prostatic urethral stent designed to gently open up the obstructed prostate and relieve lower urinary tract symptoms associated with BPH

SMART Launches Research Centre to Develop Wearable Ultrasound Imaging System
The Wearable Imaging for Transforming Elderly Care (WITEC) collaborative research project aims to develop the world’s first wearable ultrasound imaging system for continuous, real-time monitoring and personalised diagnosis of chronic conditions such as hypertension and heart failure.

3D-printed scaffolds for blood vessels point to new approach for heart bypass grafts
The tiny opaque tube that Yonghui Ding holds up to the light in his laboratory looks like a bit of debris from a dismantled ballpoint pen.
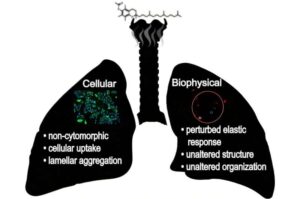
Neutron scattering sheds light on lung injuries linked to vaping
Researchers from the University of Windsor are using neutrons at the Department of Energy’s Oak Ridge National Laboratory to better understand symptoms associated with e-cigarette/vaping-associated lung injury (EVALI).
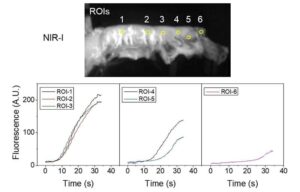
For the first time, next-gen infrared fluorescence imaging helps surgeons see blood perfusion during esophageal surgery
A cross-disciplinary research team has, for the first time, successfully applied NIR-II (1,000–3,000 nm) fluorescence video imaging during esophagectomy.
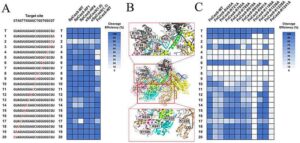
Ultrasensitive liquid biopsy method detects low-frequency cancer mutations
Liquid biopsy is increasingly recognized as a promising tool for cancer detection and treatment monitoring, yet its effectiveness is often limited by the extremely low levels of tumor-derived DNA circulating in the blood.
