MedTech News
.................... by Andrew Celentano
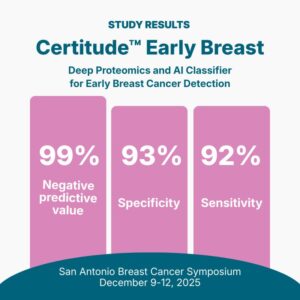
Astrin Biosciences Launches Certitude™ Breast, the First Non-Imaging Breast Cancer Detection Test
ST. PAUL, Minn., Dec. 3, 2025 /PRNewswire/ — Astrin Biosciences, a cancer intelligence company transforming how cancer is detected and treated through deep proteomics and AI, announced the launch of Certitude™, the first-of-its-kind, non-imaging, blood-based early cancer detection test. Results from the latest study will be presented at the upcoming San Antonio Breast Cancer Symposium (SABCS), taking place December 10–14, 2025.
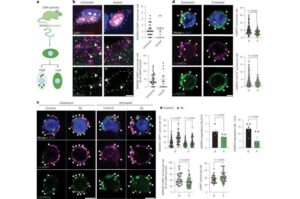
Age-related muscle wasting tied to cell recycling defect
Two related studies published today in Nature Metabolism show that a specialized intracellular recycling mechanism—chaperone-mediated autophagy (CMA)—is essential for muscle health.
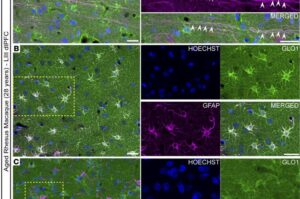
How the brain protects itself from Alzheimer’s disease
High levels of calcium are toxic to cells and contribute to loss of neurons in Alzheimer’s disease. A new study published in JCI Insight identifies a mechanism through which the young brain protects itself against high calcium levels, and it could help scientists learn how to protect the brain from this devastating neurodegenerative condition.
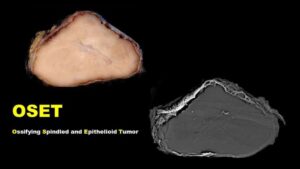
Newly defined benign soft tissue tumor with bony shell may mimic malignancy
It’s not often that a pathologist gets to make a diagnosis that works for the patient by preventing treatment from occurring.
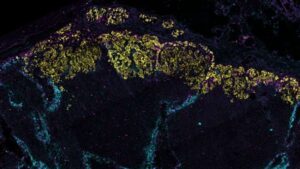
Breast cancer remodels lymphatic vessels to accelerate its spread, research reveals
Breast cancer is able to modify the lymphatic vessels through which it travels to the draining lymph nodes
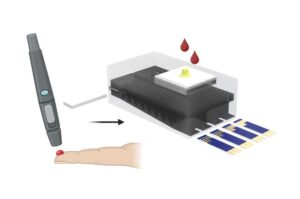
New test distinguishes vaccine-induced false positives from active HIV infection
Since the human immunodeficiency virus (HIV) was identified in 1983, roughly 91.4 million people around the world have contracted the virus and an additional 44.1 million have died from related causes.

New vaccine sparks hope in whooping cough control
A human challenge trial has shown a new vaccine could offer better protection against whooping cough.
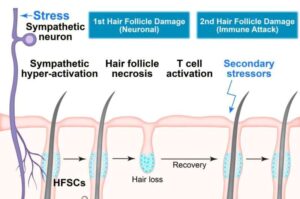
Why stress can make your hair fall out: A two-part reaction
It’s well known that stress can trigger hair loss. A new paper explores how this happens and how our response to stress can have long-term consequences for our scalps, research that may eventually yield insights into autoimmune diseases.
