MedTech News
.................... by Andrew Celentano
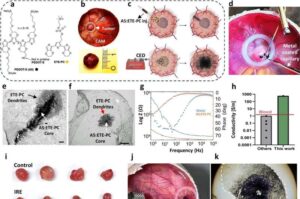
Electrotherapy using injectable nanoparticles offers hope for glioblastoma treatment
Electrotherapy using injectable nanoparticles delivered directly into the tumor could pave the way for new treatment options for glioblastoma, according to a new study from Lund University in Sweden.
Philips unveils BlueSeal Horizon helium-free 3.0T MRI platform
Major technology breakthrough combines advanced AI for clinical insights and accelerated workflow in new premium 3.0T MRI platform

MediView Achieves First CE Mark, Bringing Augmented Reality to European Healthcare
CLEVELAND, Dec. 1, 2025 /PRNewswire/ — MediView XR, Inc., a pioneer in clinical augmented reality and spatial computing, proudly announced its first CE mark certification, a significant regulatory milestone that enables the introduction of its innovative mixed reality solutions across Europe.

Rad AI Unveils Next-Generation Speech Recognition That Redefines Radiology Reporting
CHICAGO, Dec. 1, 2025 /PRNewswire/ — Rad AI, the leader in AI-powered radiology workflow solutions, today announced the launch of next-generation speech recognition technology (patent pending) that dramatically improves the speed and accuracy of diagnostic reporting. Integrated into Rad AI Reporting, the new capabilities deliver unprecedented speed and accuracy, setting a new standard for dictation in radiology.
ScreenPoint Medical Enables Transpara® Breast AI within Precision Imaging Network, part of Microsoft for Healthcare
NEW YORK and NIJMEGEN, Netherlands, Dec. 1, 2025 /PRNewswire/ — ScreenPoint Medical today announced the completion of a commercial agreement making its Transpara® breast-imaging AI portfolio available through Precision Imaging Network, part of Microsoft for Healthcare. By integrating Transpara with Precision Imaging Network , ScreenPoint Medical expands how healthcare providers can access Breast AI tools to support workflow efficiency and clinical decision-making.

NervGen’s Peptide Achieves Nervous System Repair in Phase II Spinal Cord Injury Study
NervGen will meet with the FDA early next year to align on a regulatory path forward for NVG-291 in chronic spinal cord injury.

Advanced CEM tracking enables more comprehensive breast cancer detection and care coordination
DALLAS, Nov. 30, 2025 /PRNewswire/ — Ikonopedia, a leader in breast imaging reporting and tracking systems, today announced the addition of Contrast-Enhanced Mammography (CEM) to its integrated reporting platform. This expansion enables breast imaging centers to offer patients a powerful diagnostic alternative that improves cancer detection, particularly in women with dense breast tissue. Speak with the Ikonopedia team at RSNA in booth #7803.
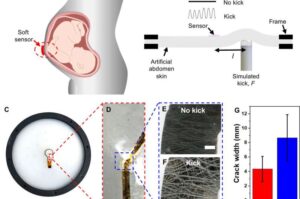
Stick-on patch can monitor a baby’s movements in utero
Engineers and obstetricians at Monash University have invented a wearable Band-Aid-like patch to track a baby’s movements through the mother’s abdomen, offering a new way to support safer pregnancies from home.
