MedTech News
.................... by Andrew Celentano
ScreenPoint Medical Enables Transpara® Breast AI within Precision Imaging Network, part of Microsoft for Healthcare
NEW YORK and NIJMEGEN, Netherlands, Dec. 1, 2025 /PRNewswire/ — ScreenPoint Medical today announced the completion of a commercial agreement making its Transpara® breast-imaging AI portfolio available through Precision Imaging Network, part of Microsoft for Healthcare. By integrating Transpara with Precision Imaging Network , ScreenPoint Medical expands how healthcare providers can access Breast AI tools to support workflow efficiency and clinical decision-making.

NervGen’s Peptide Achieves Nervous System Repair in Phase II Spinal Cord Injury Study
NervGen will meet with the FDA early next year to align on a regulatory path forward for NVG-291 in chronic spinal cord injury.

Advanced CEM tracking enables more comprehensive breast cancer detection and care coordination
DALLAS, Nov. 30, 2025 /PRNewswire/ — Ikonopedia, a leader in breast imaging reporting and tracking systems, today announced the addition of Contrast-Enhanced Mammography (CEM) to its integrated reporting platform. This expansion enables breast imaging centers to offer patients a powerful diagnostic alternative that improves cancer detection, particularly in women with dense breast tissue. Speak with the Ikonopedia team at RSNA in booth #7803.
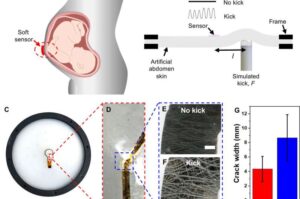
Stick-on patch can monitor a baby’s movements in utero
Engineers and obstetricians at Monash University have invented a wearable Band-Aid-like patch to track a baby’s movements through the mother’s abdomen, offering a new way to support safer pregnancies from home.

Key biological marker into why young people self-harm uncovered
As many as one in six teenagers have self-harmed at some point in their lives. As well as being an indicator of emotional pain, self-harm is also the best-known predictor of death by suicide—yet researchers know little about the emotional and biological factors that lead to it.

Prototype device restores lost smell by teaching the brain to feel odors
There is new hope for people who have lost their smell. Scientists have successfully tested a breakthrough device that lets people detect the presence of certain odors. This innovative system helps them “smell” again by translating odors into feelings (like touch) inside the nose.
a2z Radiology AI receives FDA clearance for abdomen-pelvis CT device
The a2z-Unified-Triage device is designed to simultaneously flag and prioritise seven urgent findings on abdomen-pelvis CT scans.

Epileptic seizure alert wearable set to launch in Europe in 2026
Commercialisation efforts for mjn-neuro’s EPISERA sensor in Europe will be handled by Neuraxpharma.
