MedTech News
.................... by Andrew Celentano
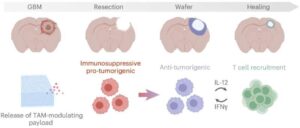
Implantable ‘CANDI’ wafer shows promise for preventing glioblastoma recurrence
Now, researchers have developed a biodegradable implant that can be placed directly into the brain cavity after tumor removal
MAGIC: AI-assisted ‘laser tag’ illuminates cancer origins
Chromosomal abnormalities—numerical and structural defects in chromosomes—are a common first step in this process, often contributing to normal cells turning cancerous.
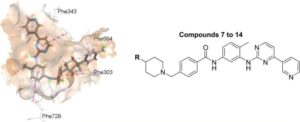
New technology restores chemotherapy effectiveness against resistant cancer cells
Scientists from King’s College London have successfully applied a new technology that disarms one of the most potent weapons cancer cells use to weaken the effects of chemotherapy drugs.
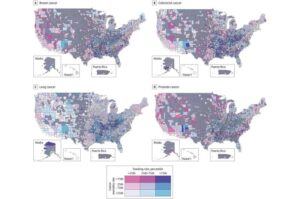
Online tool identifies and ranks community factors that predict cancer disparities
A new study published in JAMA Network Open by the Harvey L. Neiman Health Policy Institute reveals poverty, environmental risks, housing issues, and physical inactivity are top-ranking community-level predictors of disparities in cancer screening, prevalence, and deaths across U.S. counties.
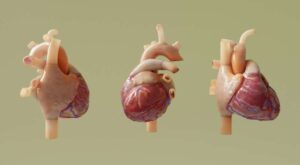
Computational model helps personalize neurostimulation therapy for atrial fibrillation
Atrial fibrillation (AFib) is a cardiac disorder in which the chambers of the heart beat rapidly and irregularly. It’s the most common type of arrhythmia and the leading cardiac cause of stroke.
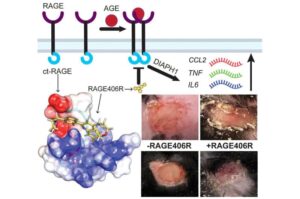
Chemists design candidate drug against diabetes
Researchers from the University at Albany and NYU Grossman School of Medicine have found a way to block a key cellular pathway known to drive chronic inflammation and impaired wound healing in people with diabetes.

Capturing cancer cells from blood could help doctors choose the right breast cancer treatment
Doctors may be able to spare patients unnecessarily aggressive breast cancer treatments by collecting and testing cancer cells in patients’ blood, research from the University of Michigan and the University of Kansas suggests.
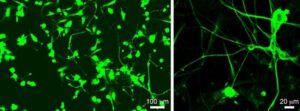
Bioinformatics uncovers regenerative therapy for spinal cord injury
Spinal cord injury (SCI) remains a major unmet medical challenge, often resulting in permanent paralysis and disability with no effective treatments. Now, researchers at University of California San Diego School of Medicine have harnessed bioinformatics to fast-track the discovery of a promising new drug for SCI.
