MedTech News
.................... by Andrew Celentano

Avicenna.AI Launches AVI Seamless Platform for Medical Imaging AI
New platform is designed to enable fast, scalable access to a wide range of AI imaging solutions

FDA grants label expansion for BrainsWay Deep TMS to treat MDD
The clearance is based on data that included real-world evidence from 1,120 adolescents who received treatment at US TMS centres.

Renu MedTech introduces tubeless insulin patch pump in India
Renu MedTech today unveiled InsuPatch, its tubeless insulin patch pump designed specifically for advancing diabetes care in India.
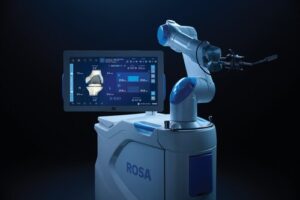
Zimmer Biomet Receives U.S. FDA Clearance for Enhanced Version of ROSA® Knee Robotic Technology
WARSAW, Ind., Nov. 14, 2025 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global medical technology leader, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of ROSA® Knee with OptimiZe™, an enhanced version of its ROSA® Knee System that offers a more customized experience for surgeons to help deliver accurate and reproducible outcomes1 in robotic-assisted total knee replacement surgery.

Overlooked layer of DNA may explain disease risk, severity
Scientists at The Hospital for Sick Children (SickKids) have revealed a previously overlooked layer of genetic variation that could help explain why people experience disease differently, and why some treatments work better for certain populations.

FDA clears Augmedics’ AR headset for use with spine surgery system
The company will introduce the headset at the North American Spine Society Annual Meeting in the US on 14 November.
FDA clears ViTAA Medical’s AI aortic surgery planning tool
ViTAA’s FDA clearance on its AiORTA tool represents the first part of its planned full-suite aortic care platform.
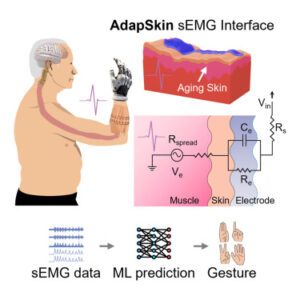
Age-adaptive polymeric skin electronics enhance neural data and machine learning accuracy
Skin-interfaced bioelectronics can help enhance health monitoring and early disease prediction. However, biomechanical and compositional differences in skin, such as dryness, wrinkling, and thinning due to the loss of collagen, can alter skin electrical impedance and conductance, compromising the functionalities of these devices.
