MedTech News
.................... by Andrew Celentano
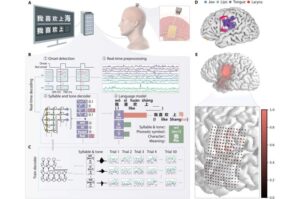
Brain-computer interface decodes Mandarin from neural signals in real time
Researchers in Shanghai have reported in a study, recently published in Science Advances, that they’ve successfully decoded Mandarin Chinese language in real time with the help of a brain-computer interface (BCI) framework.
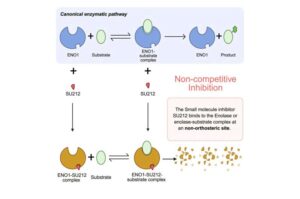
Promising drug can inhibit aggressive breast cancer
New research reveals a drug developed by scientists at Oregon Health & Science University may develop into a new treatment for an especially aggressive form of breast cancer.
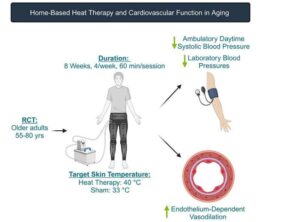
Hot pants for good health: Scientists try heat therapy to lower blood pressure
The saying goes that you should stay out of the kitchen if you can’t take the heat, but new research suggests otherwise—for the sake of your blood pressure.
Particles that enhance mRNA delivery could reduce vaccine dosage and costs
Using these nanoparticles to deliver a flu vaccine, researchers observed an effective immune response at a much lower dose.
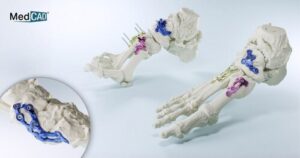
MedCAD’s AccuStride™ Fixation Plates Receive 510(k) FDA Clearance to Complete Foot and Ankle System Offering
DALLAS, Nov. 6, 2025 /PRNewswire/ — Dallas-based MedCAD has been awarded 510(k) FDA clearance for its AccuStride fixation plates, part of a patient-specific solution available to surgeons as a complete foot and ankle (F&A) system. The company received 510(k) FDA clearance for its foot and ankle guides and planning system in March 2025. The AccuStride implant and surgical guide system coupled with the company’s proprietary planning software will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.

Nitinotes Receives CE Mark Approval of EndoZip™, the First Fully Automated Suturing System for Endoscopic Sleeve Gastroplasty
CAESAREA, Israel, Nov. 6, 2025 /PRNewswire/ — Nitinotes Ltd., a medical device company transforming the treatment of obesity, today announced it has received CE Mark approval for the EndoZip™ System, the first fully automated suturing platform for endoscopic sleeve gastroplasty (ESG). The milestone clearance enables Nitinotes to begin commercialization across the European Union and other CE Mark-accepting markets.

Microfluidic sensors enable real-time sweat analysis
Eccrine sweat is a water-like fluid secreted by eccrine sweat glands that comprises various kinds of biochemical components such as electrolytes, metabolites, organic molecules, and drugs.

Review finds no link between acetaminophen use in pregnancy and neurodevelopmental disorders
A rigorous systematic review of the present state of knowledge on the use of acetaminophen during pregnancy and the risk of specific neurodevelopmental disorders (NDDs), such as autism and ADHD, offers reassurance that acetaminophen does not increase the risk of NDDs.
