MedTech News
.................... by Andrew Celentano
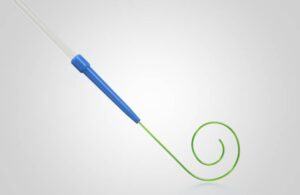
Medtronic launches new guidewire to support TAVR procedures
Medtronic (NYSE: MDT)+
today announced the launch of its Stedi extra support guidewire for use with its Evolut TAVR platform.
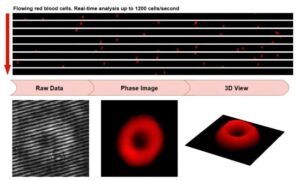
Embedded GPU platform powers real-time blood cell imaging and analysis
Blood tests are among the most common tools in medicine. Scientists are working to make blood cell imaging faster and more intuitive so that doctors can make fast and accurate diagnostic decisions.

YorLabs Announces FDA 510(k) Clearance of its Zero-CapEx Intracardiac Imaging System
BEAVERTON, Ore., Oct. 24, 2025 /PRNewswire/ — YorLabs, Inc., a medical technology company pioneering next-generation intracardiac imaging solutions for electrophysiology (EP) and interventional cardiology (IC) procedures, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the company’s YorLabs Intracardiac Imaging System – a first-of-its-kind, zero-capex ultrasound platform designed to simplify workflow, reduce cost, and enhance procedural efficiency inside the cath lab.

Alife Health’s AI embryo selection tool gains CE mark
The tool integrates with the current laboratory infrastructure, captures images of embryos and creates an AI score.

PhysioBiometrics launches Heel2Toe device to enhance gait in older adults
The device has shown to be advantageous for individuals with neurological or orthopaedic disorders.

FDA clears NeurAxis’ neurostimulator for abdominal pain
The expanded FDA clearance makes NeurAxis’ IB-Stim the first FDA approval for a treatment that specifically addresses functional dyspepsia.
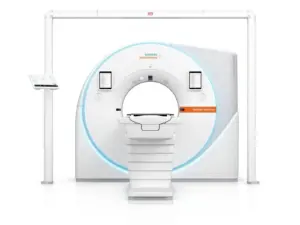
Siemens Healthineers Presents Photon-Counting CT Scanner Designed for Radiation Therapy
Novel technology delivers enhanced image contrast and resolution for confident decisions
Major study examines endoscopies that fail to detect esophageal cancer
An endoscopy—using a fiber‐optic tube to peer inside the body and collect biopsy samples—can be an invaluable way to detect cancer of the esophagus. But sometimes, an endoscopy can miss esophageal cancer, which doesn’t get detected until weeks or months later.
