
Novocure wins FDA approval to treat pancreatic cancer with electric fields
Winning approval to target the 15,000 U.S. patients with locally advanced pancreatic cancer is the first step in a broader expansion in the tumor type.

Winning approval to target the 15,000 U.S. patients with locally advanced pancreatic cancer is the first step in a broader expansion in the tumor type.

HERAKLION, Greece, Feb. 13, 2026 /PRNewswire/ — EYE PCR announces that its fixOflex endocapsular device has received CE Mark certification under the European Union Medical Device Regulation (EU MDR 2017/745), enabling commercialization in Europe and other markets that recognize the CE Mark. This regulatory milestone validates the device’s safety and efficacy, positioning EYE PCR for controlled market introduction.
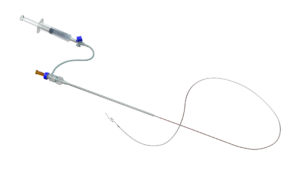
GALWAY, Ireland, Feb. 13, 2026 /PRNewswire/ — InVera Medical, an Irish medical technology company, has received European regulatory approval for a new minimally invasive device designed to help physicians deliver treatment more effectively to diseased leg veins, including varicose veins.
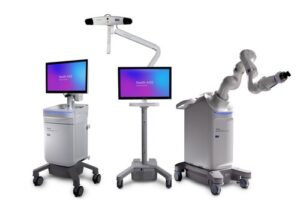
GALWAY, Ireland, Feb. 13, 2026 /PRNewswire/ — Medtronic (NYSE: MDT), a global leader in healthcare technology, today announced U.S. Food and Drug Administration (FDA) clearance of the Stealth AXiS™ surgical system, a next-generation platform that brings planning, navigation, and robotics together into a single, intelligent system for spine surgery.

Encora Therapeutics announced today that it received FDA 510(k) clearance for its Encora X1 device.

Novocure (Nasdaq:NVCR) announced that the FDA granted approval for its Optune Pax treatment for advanced pancreatic cancer.

BURLINGTON, N.C., Feb. 11, 2026 /PRNewswire/ — Labcorp (NYSE: LH), a global leader of innovative and comprehensive laboratory services, announced today the nationwide availability of the Elecsys® pTau-181 test, the first and only blood test cleared by the U.S. Food and Drug Administration (FDA) to aid in the initial assessment of Alzheimer’s disease in the primary care setting. This launch further expands Labcorp’s comprehensive portfolio of Alzheimer’s disease blood tests, offering clinicians solutions across both primary and specialty care settings.
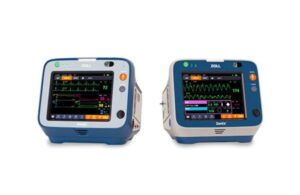
Zoll announced today that it received CE mark approval under the European Medical Device Regulation (MDR) for its Zenix monitor/defibrillator.

PLANO, Texas, Feb. 10, 2026 /PRNewswire/ — Vesalio, a global leader in vascular intervention, today announced CE Mark certification and the European commercial launch of two new neurovascular devices: NeVa™ VS, for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage (aSAH), and the NeVa™ 3.0 mm Thrombectomy System for stroke. In addition, the Company received an additional U.S. Food and Drug Administration (FDA) 510(k) clearance expanding the indications of its neurovascular and peripheral aspiration catheters to include distal access.
ANDOVER, Mass., Feb. 9, 2026 /PRNewswire/ — TransMedics Group, Inc. (“TransMedics”) (Nasdaq: TMDX), a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart, and liver failure, today announced that the U.S. Food and Drug Administration (FDA) has granted full approval of its Investigational Device Exemption (IDE) for the Next-Generation OCS ENHANCE Heart trial.