
SimBioSys Earns Third FDA Clearance for TumorSight™ Viz
AI-Powered 3D Surgical Planning Tool Advances Precision Breast Cancer Surgery with Latest Regulatory Milestone

AI-Powered 3D Surgical Planning Tool Advances Precision Breast Cancer Surgery with Latest Regulatory Milestone

Intuitive Surgical (Nasdaq: ISRG)+
announced today that the FDA cleared the latest advanced energy instrumentation for its da Vinci systems.

JERSEY CITY, N.J. and OR YEHUDA, Israel, July 10, 2025 /PRNewswire/ — Synchrony Medical, a medical technology company dedicated to advancing respiratory care, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its LibAirty™ Airway Clearance System in the United States.
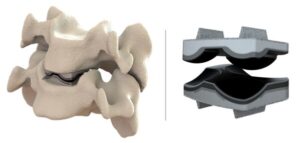
OREM, Utah, July 10, 2025 /PRNewswire/ — Dymicron®, a privately held medical device company pioneering advanced spinal technologies, today announced that the United States (U.S.) Food and Drug Administration (FDA) has granted Investigational Device Exemption (IDE) approval to begin a pivotal clinical study of the Triadyme®–C cervical artificial disc.
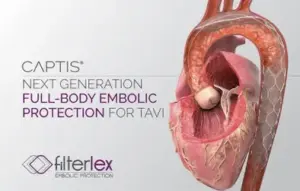
The FDA has granted 510(k) clearance to the TaviPilot AI software developed by Caranx Medical, according to company officials.
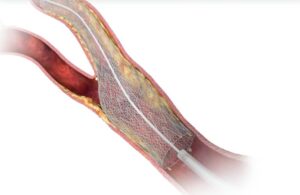
InspireMD (Nasdaq:NSPR) announced today that it launched its CGuard Prime carotid stent in the U.S. after receiving FDA premarket approval (PMA) last month.

NEUCHÂTEL, Switzerland, July 9, 2025 /PRNewswire/ — Aktiia, a pioneer in optical blood pressure monitoring, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for over-the-counter (OTC) use of its cuffless blood pressure monitoring technology.
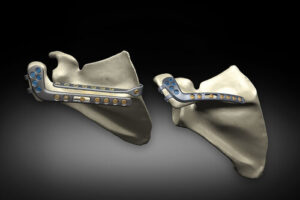
GAINESVILLE, Fla., July 9, 2025 /PRNewswire/ — Exactech, a global medical technology leader, announced the Equinoxe® Scapula Reconstruction System for acromial and scapular spine fractures has received 510(k) clearance1 from the U.S. Food and Drug Administration, marking a significant advancement in the treatment of acromial and scapular spine fractures.

Oxiplex is the only FDA authorized intraoperative gel indicated as an adjunct to lumbar spinal surgery to reduce postoperative leg pain and neurological symptoms.
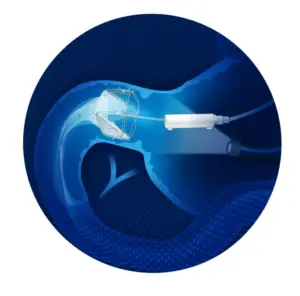
Regulatory milestone paves the way for expanded access to Morphic’s non-implant, incisionless solution for treating acid reflux and related gastrointestinal disorders across Europe.