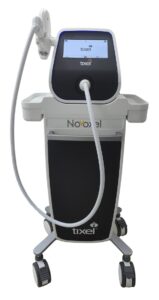
Novoxel Inc. announces the U.S. FDA clearance of the Tixel i (pronounced “Tixel Eye”) for sale in the USA.
Simple Relief™ from Dry Eye comes from 2-minute treatment with Tixel i®

Simple Relief™ from Dry Eye comes from 2-minute treatment with Tixel i®

FREMONT, Calif., Nov. 18, 2024 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with the LinkSymphoKnee (LSK) from Waldemar Link GmbH & Co. KG, Germany (LINK) under a Collaboration Agreement between the two companies.
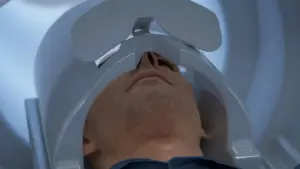
The system is designed to shorten scan times, which may be better for people who struggle to stay still or have claustrophobia, and to detect more subtle abnormalities.

EINDHOVEN, The Netherlands, Nov. 14, 2024 /PRNewswire/ — Xeltis, a leading developer of transformative implants that enable the natural creation of living and long-lasting vessels, today announces that the Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) has granted aXess Breakthrough Device Designation status. aXess is Xeltis’ vascular access conduit, which enables the creation of a new, permanent, living vessel for hemodialysis vascular access.
BOSTON and LEUVEN, Belgium, Nov. 14, 2024 /PRNewswire/ — Why this news will impact the lives of people affected by Alzheimer’s disease:

HOFFMAN ESTATES, Ill., Nov. 14, 2024 /PRNewswire/ — In a pivotal stride to address the growing AFib epidemic, OMRON Healthcare today announced the U.S. Food and Drug Administration (FDA) has granted the company its De Novo authorization to market new home blood pressure monitors featuring breakthrough AI-powered atrial fibrillation detection. In a medical device first, OMRON’s novel machine learning IntelliSense™ AFib algorithm automatically analyzes the Pressure Pulse Wave generated during blood pressure measurement to detect AFib, a leading cause of stroke.
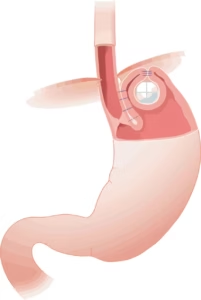
VADUZ, Liechtenstein, Nov. 14, 2024 /PRNewswire/ — Implantica AG (publ.), a medtech company at the forefront of introducing advanced technology into the body, including the unique device RefluxStop™ for the treatment of acid reflux, a treatment field with 1 billion sufferers, announces the submission of the second clinical module of the Premarket Approval (PMA) application to the US FDA for RefluxStop™ along with responses to the FDA’s findings from the first module.
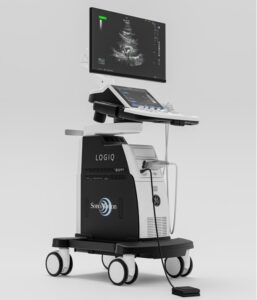
SAN MATEO, Calif., Nov. 13, 2024 /PRNewswire/ — SonoMotion, a medical device company developing non-invasive solutions for kidney stones, announced today that the FDA has granted de novo clearance for the Company’s Stone Clear™ device for the anesthesia-free treatment of post-lithotripsy kidney stone fragments.
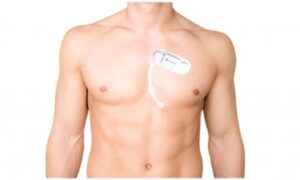
LAUSANNE, Switzerland, Nov. 13, 2024 /PRNewswire/ — SmartCardia has received FDA clearance for Mobile Outpatient Cardiac Telemetry (OCT/MCT) for its 7-lead live ECG monitoring patch and cloud platform. SmartCardia’s 7L patch is easy-to-wear, cable-free, waterproof and can be used for continuous monitoring for up to 14 days
Johnson & Johnson MedTech announced today that the FDA granted its Ottava surgical robot investigational device exemption (IDE).