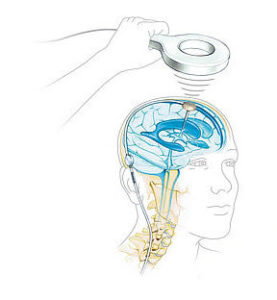
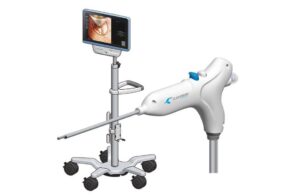
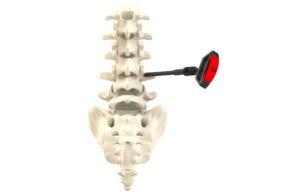
CENTER VALLEY, Pa., Aug. 27, 2024 /PRNewswire/ — Aesculap, Inc. (Aesculap), in partnership with Christoph Miethke GmbH & Co. KG (MIETHKE), announced today that the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation for the M.scio® System. This unique, non-invasive, telemetric pressure measurement system is designed to provide continuous access to long-term, intracranial pressure (ICP) monitoring of cerebrospinal fluid (CSF) for the management of hydrocephalus via a permanent, fully implantable sensor.