Scientists discover natural ‘brake’ that could stop harmful inflammation
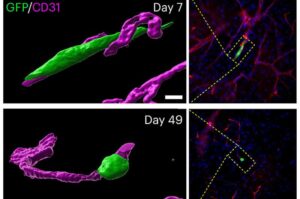
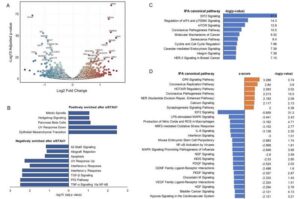
Researchers at University College London (UCL) have uncovered a key mechanism that helps the body switch off inflammation—a breakthrough that could lead to new treatments for chronic diseases affecting millions worldwide.