
FDA accepts MannKind sNDA for autoinjector that treats edema
MannKind (Nasdaq:MNKD) announced that the FDA accepted a supplemental New Drug Application (sNDA) for its Furoscix ReadyFlow autoinjector.

MannKind (Nasdaq:MNKD) announced that the FDA accepted a supplemental New Drug Application (sNDA) for its Furoscix ReadyFlow autoinjector.

The decision is supported by evidence from a clinical study and data from supporting analytical validation trials.

A new analysis of research into the most common type of breast cancer has zeroed in on an overlooked hormone that may be responsible for the increased risk of breast cancer death in postmenopausal women with obesity.

Bill Janes is on a mission to improve life for people with amyotrophic lateral sclerosis (ALS). As a licensed occupational therapist and researcher at the University of Missouri, he’s seen firsthand how the disease can steal a person’s strength, speech and independence.
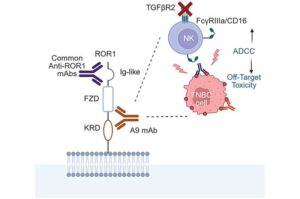
A cancer-targeting antibody that helps the body’s immune cells spot and destroy hard-to-treat tumors such as triple-negative breast cancer has been developed by researchers.

Scientists at The University of Texas at El Paso have found a promising new target in the fight against high-grade serous carcinoma, an aggressive form of ovarian cancer. Less than 50% of women survive five years after diagnosis, according to the team.
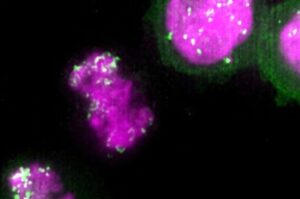
Small, cancer-associated DNA circles “hitchhike” on chromosomes during cell division to spread efficiently to daughter cells by co-opting a process used to maintain cellular identity through generations, Stanford Medicine-led research has found.
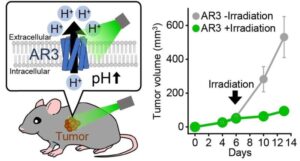
One of the hallmarks of cancer cells is their ability to evade apoptosis, or programmed cell death, through changes in protein expression.
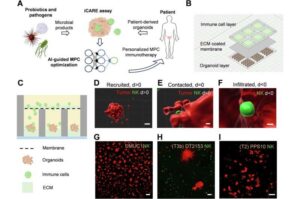
Bacteria may be the next frontier in cancer treatment, according to a team led by researchers at Penn State that devised a new approach of creating bacteria-derived mixtures—or cocktails—to help fight bladder cancer.
A team of scientists have developed a simple method for automated manufacturing of lung organoids which could revolutionize the development of treatments for lung disease. These organoids, miniature structures containing the cells that real lungs do, could be used to test early-stage experimental drugs more effectively, without needing to use animal material.