MedTech News
.................... by Andrew Celentano
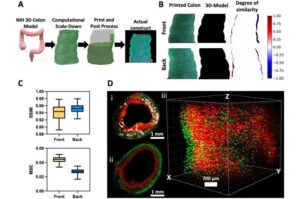
Bioelectronic-integrated artificial colon eliminates need for animal testing
Researchers at the University of California, Irvine have developed a 3D human colon model integrated with bioelectronics to aid in colorectal cancer research and drug discovery. The “3D in vivo mimicking human colon” enables precision, personalized medicine and offers a more ethical, accurate and cost-effective alternative to traditional animal testing.
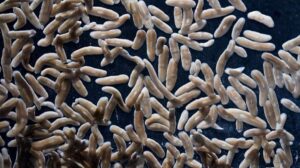
Tiny regenerative worm offers insights into tissue repair and regenerative medicine
Stem cells in most organisms typically take cues from adjacent cells. But new research from the Stowers Institute for Medical Research reveals planarian stem cells ignore their nearest neighbors and instead respond to signals farther away in the body. This discovery may help explain the flatworm’s extraordinary ability to regenerate—and could offer clues for developing new ways to replace or repair tissues in humans.
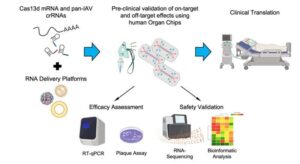
Human organ chip technology sets stage for pan-influenza A CRISPR RNA therapies
The influenza A virus (IAV) has been the cause of six major flu pandemics, responsible for 50 to 100 million deaths globally. In the U.S. alone, it is estimated that despite seasonally updated vaccines, IAV infections still lead to 140,000 to 710,000 hospitalizations and 12,000 to 52,000 deaths annually.
Stereotaxis wins CE mark for Synchrony system, submits to FDA
Stereotaxis (NYSE:STXS) announced today that it received CE mark for its Synchrony system while also submitting it to the FDA for 510(k) clearance.
NextBiomedical begins US pivotal trial for Nexsphere-F knee pain device
NextBiomedical this week announced it enrolled the first patient in its U.S. pivotal clinical trial evaluating its Nexsphere-F, a fast-resorbable microsphere for embolization treatment of musculoskeletal pain.
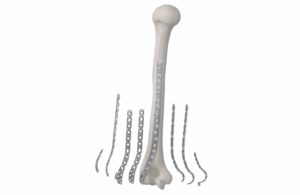
Globus Medical launches Anthem Elbow Fracture System
Globus Medical has launched the Anthem Elbow Fracture System, a plating system designed to help surgeons manage a wide range of elbow fractures with simplified workflow and flexible fixation options.
Vara earns CE mark for AI breast imaging
Vara has announced that it has received a new CE certificate for its new breast-imaging AI.
Stereotaxis teams with CardioFocus to develop robotic PFA system
https://www.medtechdive.com/news/stereotaxis-teams-with-cardiofocus-to-develop-robotic-pfa-system/802799/
