MedTech News
.................... by Andrew Celentano
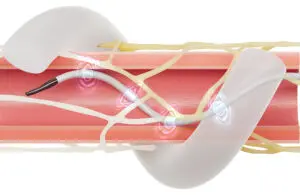
Medtronic Symplicity Spyral renal denervation tech wins regulatory nod in Japan
Medtronic (NYSE: MDT)+
announced that it received regulatory approval in Japan for its Symplicity Spyral renal denervation (RDN) system.
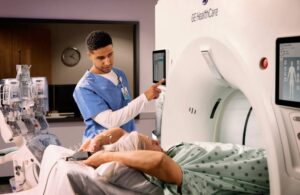
GE HealthCare wins FDA clearance for new coronary CT system
GE HealthCare (Nasdaq: GEHC)+
announced that it received FDA 510(k) clearance for its Revolution Vibe CT imaging system.

China-based Surgerii Robotics wins CE mark for single-port surgical robot
Surgerii Robotics announced on LinkedIn today that its Shurui single-port surgical robot garnered CE mark approval in Europe.
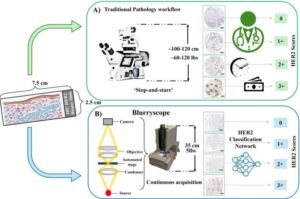
BlurryScope: A compact, AI-powered microscope for rapid, cost-effective cancer scoring
A research team at UCLA, led by Professor Aydogan Ozcan, has introduced BlurryScope, a compact, cost-effective scanning microscope that combines simple optical hardware with advanced deep learning algorithms to assess HER2 status in breast cancer tissue samples.

WiFi signals can measure heart rate—no wearables needed
Heart rate is one of the most basic and important indicators of health, providing a snapshot into a person’s physical activity, stress and anxiety, hydration level, and more.

Widely available nasal spray reduces risk of coronavirus infection by two-thirds, clinical study suggests
A research team at Saarland University has demonstrated in a clinical study that a widely used anti-allergy nasal spray containing the active ingredient azelastine can significantly reduce the risk of infection with the SARS-CoV-2 virus. The results of the placebo-controlled trial involving 450 healthy participants have now been published in JAMA Internal Medicine.
FDA grants 510(k) clearance to 4DMedical’s CT:VQ for respiratory diagnostics
The submission for CT:VQ was supported by a clinical validation package that spanned a variety of lung conditions.
Phraxis Announces First-Ever Commercial Case of EndoForce Anastomotic Connector
The technology is compatible with all PTFE grafts and was studied extensively in the company’s pivotal clinical trial
