MedTech News
.................... by Andrew Celentano

P&F Secures EU MDR CE Certification for TricValve® System
VIENNA, Feb. 5, 2026 /PRNewswire/ — P&F Products and Features GmbH, a global heart valve MedTech company focused on transcatheter solutions for structural heart disease, today announced it has received CE Mark certification under the European Union Medical Device Regulation (MDR) 2017/745 for its TricValve® Transcatheter Bicaval Valve System. The certification confirms TricValve’s compliance with the EU’s most stringent medical device regulatory requirements.
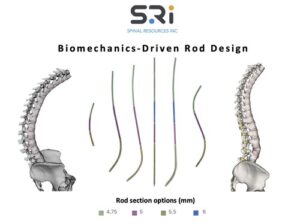
First-of-its-kind Universal Clearance Establishes Maximum Regulatory Access to the Bezier Parametric Curve Spinal Rod System for Patients and Surgeons
FORT LAUDERDALE, Fla., Feb. 5, 2026 /PRNewswire/ — The Bezier Parametric Curve Rod System from Spinal Resources, Inc. has received 510(k) clearance for compatibility with any cleared pedicle screw set available on the US market, regardless of manufacturer.
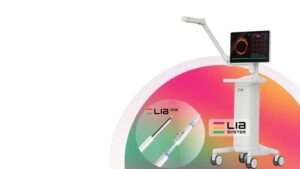
LEADOPTIK Announces First-in-Human Use of FDA-Cleared LIA™ System
SAN JOSE, Calif., Feb. 5, 2026 /PRNewswire/ — LEADOPTIK, Inc., a medical technology company focused on advancing precision lung cancer biopsy, today announced the successful first-in-human clinical use of its FDA-cleared Last Inch Assessment™ (LIA) System at University of Chicago Medical Center.

Insulet launches Omnipod 5, Omnipod Discover in the Middle East
Insulet (Nasdaq:PODD) announced today that it launched its Omnipod 5 and Omnipod Discover offerings in the Middle East.
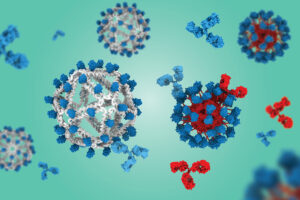
New vaccine platform promotes rare protective B cells
Based on a virus-like particle built with a DNA scaffold, the approach could generate broadly neutralizing antibody responses against HIV or influenza.
Hologic’s Aptima assay secures FDA approval for HPV primary screening
With this FDA approval, Hologic now offers three guideline-recommended methods: Pap testing, HPV primary testing, and co-testing.
UK’s NICE greenlights Abbott’s CardioMEMS heart failure sensor
The implantable blood pressure sensor will now be available to patients with chronic heart failure through the NHS.
Zimmer Biomet gets FDA nod for newest addition to G7 hip implant line
Zimmer Biomet (NYSE: ZBH)+
announced today that it received FDA 510(k) clearance for its G7 acetabular system for hip replacement surgeries.
