MedTech News
.................... by Andrew Celentano
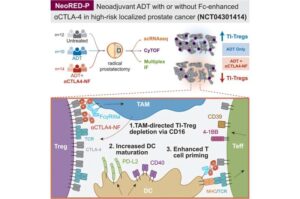
A potential immunotherapy strategy for early-stage prostate cancer
Immunotherapy has been generally ineffective for prostate cancer because the tumors are considered immunologically “cold,” meaning they do not attract enough immune cells to mount a strong attack.

First-ever in-utero stem cell therapy for fetal spina bifida repair shows safe results
A Phase I clinical trial published in The Lancet has shown that combining stem cell therapy with standard fetal surgery before birth is a safe and promising approach to treat myelomeningocele, a severe form of spina bifida.
Laser Therapy Boosts Survival in Treating Brain Cancer, With Nearly Half Alive at 18 Months
Learn how a new laser-based therapy is giving patients with aggressive brain cancer a stronger chance at survival.
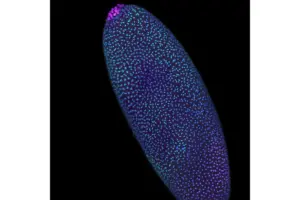
Imaging Technique Reveals DNA’s Hidden Shape in the Earliest of Embryos
Learn more about Pico-C, a tool that helps reveal the genome’s structure during the first days of life.
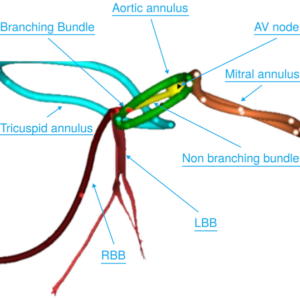
Cara Medical Receives FDA 510(k) Clearance for the CARA System for Noninvasive CTA based Cardiac Conduction System Visualization
TORTOLA, British Virgin Islands, Feb. 25, 2026 /PRNewswire/ — Cara Medical Ltd. today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the CARA System, a computed tomography angiography (CTA)-based platform that provides noninvasive, patient-specific three-dimensional (3D) visualization of the cardiac conduction system.
Scopio Labs Achieves EU IVDR Certification for AI-Powered Full-Field Digital Morphology Platforms
PARSIPPANY, N.J., Feb. 25, 2026 /PRNewswire/ — Scopio Labs today announced it has achieved IVDR certification from BSI, a major regulatory milestone that clears the path for its AI-driven digital morphology platforms in the European Union
Polaroid Therapeutics (PTx) Receives CE Mark for POLTX_Fiber™: the first application of APT™ to launch a new standard in wound care
ZURICH, Feb. 25, 2026 /PRNewswire/ — Polaroid Therapeutics (PTx) today announces that POLTX_Fiber™ has received the CE Mark as a Class IIb medical device with APT™ (Antimicrobial Polymer Technology).

4WEB Medical Receives 510(k) Clearance to Market its New SI Joint Truss System™
DALLAS, Feb. 25, 2026 /PRNewswire/ — 4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary TRUSS Implant Technology™, announced that it has received 510(k) clearance to market its SI Joint Truss System™.
