MedTech News
.................... by Andrew Celentano
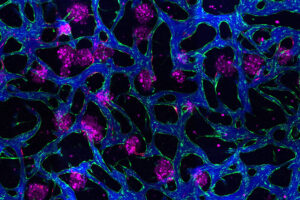
New tissue models could help researchers develop drugs for liver disease
Two models more accurately replicate the physiology of the liver, offering a new way to test treatments for fat buildup.

SMART launches new Wearable Imaging for Transforming Elderly Care research group
WITEC is working to develop the first wearable ultrasound imaging system to monitor chronic conditions in real-time, with the goal of enabling earlier detection and timely intervention.
Quest launches flow cytometry MRD test for myeloma
Quest expects the new blood test will support response monitoring in clinical trials.
FDA clears RevealDX’s AI lung nodule diagnostic
RevealDX’s software analyses CT scans and assigns lung nodules with a Malignancy Similarity Index score to aid in lung cancer diagnosis.

Johnson & Johnson MedTech announces updates to Varipulse, new ultrasound catheter
Johnson & Johnson MedTech (NYSE: JNJ)+
today announced the full commercial release of its NuVision Nav ultrasound catheter.

Test strip with enhanced technology could make way for more accessible diagnosis
A research team led by La Trobe University has developed a single-use test strip that could ultimately change how diseases like cancer are diagnosed. The research used enzymes to boost an electrical signal to detect disease-indicative molecules, also known as microRNAs.

Lab-grown heart tissue beats on its own as sensors track force in real time
Scientists at Université de Montréal and its affiliated Centre de recherche Azrieli du CHU Sainte-Justine have made a major advance in their research into cardiovascular disease: They’ve created functional, three-dimensional heart tissue that can beat autonomously in vitro.

Cynosure Lutronic secures CE Mark for Mosaic 3D platform
The Mosaic 3D device incorporates several features designed to enhance safety and patient comfort.
