MedTech News
.................... by Andrew Celentano
GE HealthCare wins FDA, CE mark approval for Allia Moveo image-guided platform
GE HealthCare (Nasdaq: GEHC)+
announced today that it received FDA 510(k) clearance and CE mark for its Allia Moveo platform.

Medtronic earns Medicare, FDA wins for MiniMed 780G with Instinct sensor, including for type 2 diabetes
Medtronic (NYSE: MDT)+
today announced three U.S. milestones expanding access to its MiniMed 780G automated insulin delivery system.

A ‘window to the brain’: Chip tracks glioblastoma treatment response using tumor vesicles in blood
Technology created at the University of Queensland could improve the odds of surviving brain cancer and change how we treat a range of neurological conditions.

A portable ultrasound sensor may enable earlier detection of breast cancer
For people who are at high risk of developing breast cancer, frequent screenings with ultrasound can help detect tumors early.

Pink noise reduces REM sleep and may harm sleep quality
Pink noise—often used to promote sleep—may reduce restorative REM sleep and interfere with sleep recovery. In contrast, earplugs were found to be significantly more effective in protecting sleep against traffic noise.

Experimental immunotherapy clears harmful artery cells, reducing plaque in mice
Scientists have designed an immunotherapy that reduces plaque in the arteries of mice, presenting a possible new treatment strategy against heart disease.

Anticipating aging-related mental decline using saliva samples and AI
As humans age beyond early adulthood, their physical and mental functions tend to slowly worsen over time. One of the most common sources of severe mental decline in older adults are neurodegenerative diseases, conditions characterized by the progressive loss of neurons in the brain or peripheral nervous system.
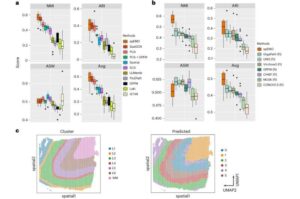
New AI tool helps scientists see how cells work together inside diseased tissue
A new study from Yale University researchers shows how artificial intelligence can bring image, gene and protein data together, offering a clearer picture of what is happening inside the body and how diseases develop.
