MedTech News
.................... by Andrew Celentano

Aidoc Secures FDA Clearance for Healthcare’s First Comprehensive Foundation Model AI
11 newly cleared indications, combined with three existing ones, introduce an AI safety net for crowded Emergency Departments and imaging backlogs
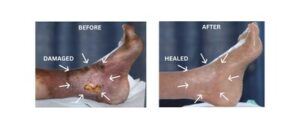
First Peer-Reviewed Study Shows Tissue Once Deemed Non-Salvageable Can Be Healed in Patients Facing Amputation
BREA, Calif., Jan. 21, 2026 /PRNewswire/ — Rapid Nexus Nanotech Wound Solutions, Inc., a California-based medical technology company, today announced the publication of a peer-reviewed study documenting outcomes that challenge long-held assumptions about neuropathy-driven tissue damage and limb loss.

Machina Medical Receives FDA Clearance for MFUSE External Bleeding Control Device
MFUSE is designed to support clinicians in managing external bleeding by forming a flexible hydrogel barrier when applied to the wound site

SurGenTec gains new FDA indication for ION-C facet fixation system
SurGenTec’s ION-C system is now indicated for the treatment of cervical pseudoarthrosis when implanted bilaterally within the facet joints.
FDA clears Cepheid’s Xpert GI Panel for pathogen detection
The Xpert GI Panel identifies pathogens directly from stool specimens collected in Cary-Blair transport media.

mOm Incubators wins FDA nod for first-of-its-kind portable incubator
mOm Incubators announced today that the FDA granted 510(k) clearance for its Essential Incubator system.
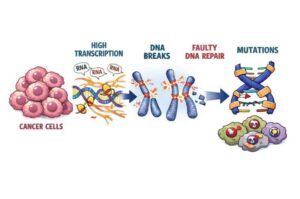
Super-enhancers in cancer cells trigger DNA breaks and error-prone repair cycles
A new study shows that cancer damages its own DNA by pushing key genes to work too hard. Researchers found that the most powerful genetic “on switches” in cancer cells, called super-enhancers, drive unusually intense gene activity. That high gear creates stress on the DNA and can cause dangerous breaks.
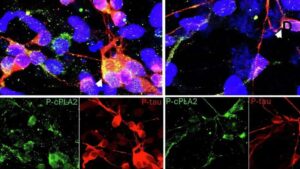
Scientists identify promising new target for Alzheimer’s-linked brain inflammation
A multidisciplinary team has developed a selective compound that inhibits an enzyme tied to inflammation in people at genetic risk for Alzheimer’s, while preserving normal brain function and crossing the blood-brain barrier.
