MedTech News
.................... by Andrew Celentano
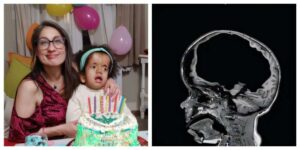
RNA therapy may be a solution for infant hydrocephalus
Hydrocephalus is a life-threatening condition that occurs in about 1 in 1,000 newborns and is often treated with invasive surgery. Now, a new study offers hope of preventing hydrocephalus before it even occurs. The paper is published in the journal Molecular Therapy.
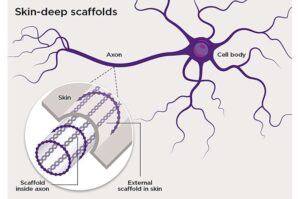
Pleasure and pain: Tiny worm reveals secret to protecting skin sensations
A tiny roundworm has helped University of Queensland scientists uncover minuscule structures in skin tissue that may protect the body’s ability to feel temperature, touch and pain. The research is published in Science Advances.

AI tool predicts six-month risks for cancer patients after heart attack
Cancer patients who suffer a heart attack face a dangerous mix of risks, which makes their clinical treatment particularly challenging. As a result, patients with cancer have been systematically excluded from many clinical trials and available risk scores. Until now, doctors had no standard tool to guide treatment in this vulnerable group.
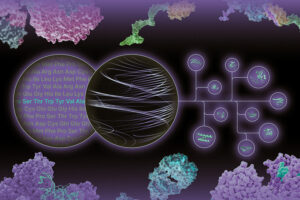
Designing the future of metabolic health through tissue-selective drug delivery
Founded by three MIT alumni, Gensaic uses AI-guided protein design to deliver RNA and other therapeutic molecules to specific cells or areas of the body.

FDA clears Spectrum Dynamics’ Veritas.AI platform
Veritas.AI is designed to advance the functionality of Spectrum Dynamics’ VERITON-CT scanner for nuclear imaging.
KORU Medical receives FDA clearance for FreedomEDGE infusion system
Rystiggo is administered weekly, 3ml to 6ml over 15 to 30 minutes for six weeks.
GRAIL Submits FDA Premarket Approval Application for the Galleri® Multi-Cancer Early Detection Test
MENLO PARK, Calif., Jan. 29, 2026 /PRNewswire/ — GRAIL, Inc. (Nasdaq: GRAL), a healthcare company whose mission is to detect cancer early when it can be cured, today announced the submission of the final module of the Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA) for its Galleri® multi-cancer early detection (MCED) test. The FDA designated the test as a Breakthrough Device in 2018.
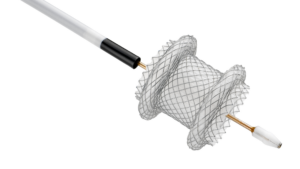
Zeus Launches PFX™ Platform and Introduces PFX Flex™ Sub-Lite-Wall™, Advancing the Future of Catheter Innovation
ORANGEBURG, S.C., Jan. 29, 2026 /PRNewswire/ — Zeus, a global leader in advanced polymer solutions, today announced the launch of PFX™, a breakthrough platform designed to advance catheter innovation with a focus on performance, design flexibility, and sustainability.
