MedTech News
.................... by Andrew Celentano

Early signs of Parkinson’s can be identified in the blood
A team led by researchers at Chalmers University of Technology, Sweden, has succeeded in identifying biomarkers for Parkinson’s disease in its earliest stages, before extensive brain damage has occurred. The biological processes leave measurable traces in the blood, but only for a limited period.
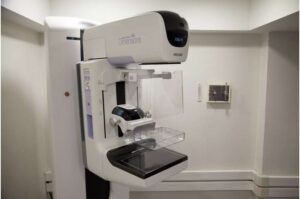
AI-supported mammography screening results in fewer aggressive and advanced breast cancers
Artificial intelligence (AI)-supported mammography identifies more cancers during screening and reduces the rate of breast cancer diagnosis by 12% in the years following, finds the first randomized controlled trial of its kind. The trial involved over 100,000 Swedish women, and its results are published in The Lancet.
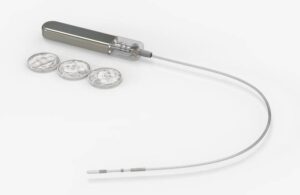
Glucotrack picks up new patents for continuous blood glucose monitor
Glucotrack (Nasdaq:GCTK) announced today that it received new U.S. patents for its continuous blood glucose monitoring (CBGM) platform.

Top medtech trends to watch in 2026
From M&A to surgical robotics and user fee negotiations, the medical device industry has a busy year ahead. Check out MedTech Dive’s roundup of the top medtech trends to watch in 2026.
RevBio receives FDA clearance for cranial bone glue trial
The FDA has authorised up to 15 clinical sites to take part in the study.

Imperative Care launches new Zoom 4S catheter
Imperative Care announced the launch of the new Zoom 4S thrombectomy catheter and reported the first patient cases with the device.

The top 10 cardio device stories of 2025
Pulsed-field ablation, transcatheter heart valves, and intravascular lithotripsy are among the top stories, as they drive growth among the largest cardio device companies.
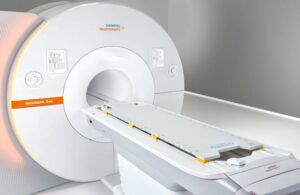
Siemens Healthineers wins FDA nod for PET/MR imaging scanner
Siemens Healthineers announced today that it received FDA clearance for its Biograph One second-generation PET/MR scanner.
