MedTech News
.................... by Andrew Celentano
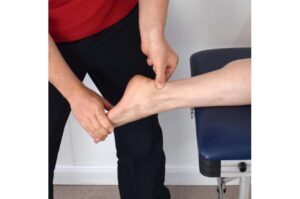
HIF1 protein identified as key trigger in common tendon diseases
Complaints such as pain in the Achilles tendon, tennis elbow, swimmer’s shoulder and jumper’s knee are familiar to many young sportspeople, as well as to older individuals. These conditions are all caused by overloading of tendons and are generally very painful.
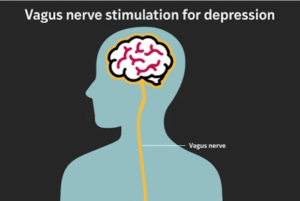
Implant provides lasting relief for treatment-resistant depression, study finds
About 20% of U.S. adults experience major depression in their lifetime. For most people, symptoms improve within a few treatment attempts, but up to one‐third of patients have treatment‐resistant depression, for which standard antidepressant medication or psychotherapy isn’t enough.

Customizable stainless steel neural probes enable safer, less expensive brain sensing
The human brain is complex. Understanding deep brain function usually requires the insertion of probes that frequently result in irreversible tissue damage. Current neural probes are made out of silicon, a brittle material that can shatter during placement.
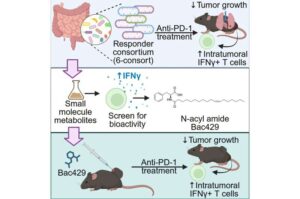
Gut bacteria molecule boosts lung cancer treatment response
UF Health Cancer Institute researchers have discovered a small compound produced naturally by gut bacteria that doubled the response to lung cancer immunotherapy treatment in mice and can now be made into a drug for testing in humans.
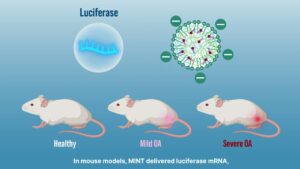
Research Spotlight: Developing “Smart” Nanoparticles to Deliver Targeted Gene Therapy in Osteoarthritis
Nanoparticles are engineered to home in on areas where cartilage has degenerated in osteoarthritis, ensuring that treatment concentrates exactly where it is needed
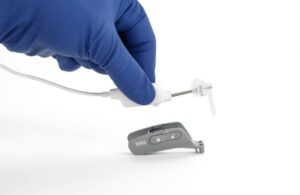
IotaMotion wins expanded FDA nod for pediatric use of robotic-assisted cochlear implant placement
IotaMotion announced today that it received FDA 510(k) clearance for expanded pediatric use of its iotaSoft insertion system.

Boston Scientific wins CE mark for Embold detachable coil system
Boston Scientific (NYSE: BSX)+
announced that it received CE mark approval for its Embold detachable coil system.
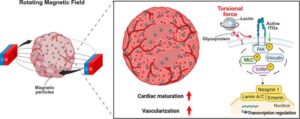
Three-dimensional magnetic torque enables heart mechanics in organoids
Heart disease remains the leading cause of death worldwide, yet progress in understanding and treating cardiac disorders is limited by the shortcomings of existing experimental models. Traditional animal models often fail to capture human-specific cardiac biology, while conventional two-dimensional cell cultures lack the functional and structural complexity of heart tissue.
