MedTech News
.................... by Andrew Celentano

Spectrumedics Announces CE Mark Approval For Its Coronary Intravascular Lithotripsy System
SINGAPORE, Jan. 6, 2026 /PRNewswire/ — Spectrumedics Medical (hereinafter “Spectrumedics”) is pleased to announce that its Sonico-CX Intravascular Lithotripsy (IVL) System has obtained CE Mark certification under the European Union Medical Device Regulation (EU MDR). The system comprises the Sonico-CX Coronary Intravascular Lithotripsy Catheter and the Intravascular Lithotripsy Generator.
Gut bacteria protect mice with influenza A from bacterial pneumonia, study finds
Select gut bacteria protect mice against post-influenza virus secondary bacterial pneumonia, according to a study published by researchers in the Institute for Biomedical Sciences at Georgia State University.

X-raying auditory ossicles: New technique reveals structures in record time
Scientists at the Paul Scherrer Institute PSI have refined an X-ray diffraction technique for detecting biological structures from nanometers to millimeters—reducing the time needed to make the measurement from around one day to about an hour. This opens up a wide range of possibilities for biomedical research—from analyzing bone and tissue structures to supporting the development of new implants.

Genes that predispose an individual to pancreatic cancer identified
A new study by the National Cancer Research Center (CNIO) has identified several sets of genes related to the predisposition to develop pancreatic ductal adenocarcinoma (the most common type of pancreatic cancer), as well as the prognosis of the disease once it has appeared.

A simple blood test can predict Crohn’s disease years before symptoms appear
Sinai Health researchers have shown a blood test that can predict Crohn’s disease years before symptoms appear, opening the doors to early diagnosis and potentially prevention.

Scientists report new immune insights and targets into LRRK2 mutations in Parkinson’s disease
Parkinson’s disease (PD) is a debilitating and progressive neurodegenerative disorder caused by the loss of dopamine-producing neurons in the substantia nigra, a brain region essential for motor control. Clinically, it is marked by tremor, rigidity, bradykinesia and postural instability, symptoms that progressively erode independence and quality of life.
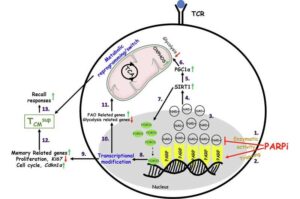
T cells gain superior memory through new reprogramming method, boosting cancer-fighting abilities
Georgetown University’s Lombardi Comprehensive Cancer Center researchers have identified a new way to reprogram T cells, which are infection and tumor-fighting white blood cells, so that they have a superior memory, thereby making them more effective in killing cancer cells.

A new tool could tell us how consciousness works
Consciousness is famously a “hard problem” of science: We don’t precisely know how the physical matter in our brains translates into thoughts, sensations, and feelings. But an emerging research tool called transcranial focused ultrasound may enable researchers to learn more about the phenomenon.
