MedTech News
.................... by Andrew Celentano

NEOS Cranial LOOP™ Receives FDA Clearance for CustomizedBone™ Use
Ross Bjella, Kelyniam’s CEO, said, “This approval will further boost Kelyniam’s sales momentum which started in Q4 last year

University of Birmingham scientists using novel hydrogel to create ‘lollipops’ for mouth cancer diagnostic
Researchers from the University of Birmingham have received funding from Cancer Research UK and the Engineering and Physical Sciences Research Council to create a new ‘lollipop’ diagnostic for mouth cancer using a novel smart hydrogel.

Fractyl Secures FDA Approval for Weight Maintenance Study After Discontinuation of GLP-1s
The FDA’s Investigational Device Exemption (IDE) clears the way for a pivotal study of Fractyl’s Revita system designed to maintain weight loss after patients have stopped taking GLP-1 drugs
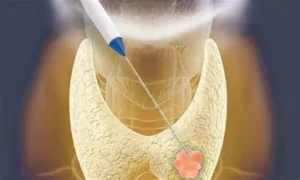
Baird Medical Granted New Class III Certificate by China’s NMPA for Ceramic Thyroid Ablation Needle
Baird Medical Devices, Inc. today announced it has been granted a new Class III certificate by the National Medical Products Administration (NMPA) in China

Roche wins FDA approval for first molecular malaria blood donor screening test
The company is pitching the test as a way to improve the safety and availability of blood.

Endostart receives FDA clearance for Endorail product to optimize colonoscopy procedural outcomes
Endorail is a magnetic balloon solution that optimises colonoscopy procedural outcomes.

Blood, Sweat, and Water: New Paper Analytical Devices Easily Track Health and Environment
Novel inexpensive, shelf-stable, easy-to-use tests bring lab-level precision to the home and field
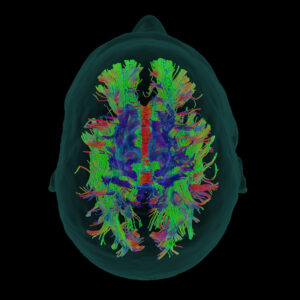
Synaptive Medical Receives FDA 510(k) Clearance for Near-Infrared Fluorescence Visualization, Expanding Application of Existing Robotic Exoscope
Near-Infrared fluorescence now available on Modus X robotic exoscope, complementing system’s existing advanced fluorescence capabilities
