MedTech News
.................... by Andrew Celentano
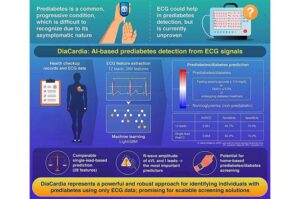
AI model detects prediabetes using ECG data without need for blood tests
DiaCardia, a novel artificial intelligence model that can accurately identify individuals with prediabetes using either 12-lead or single-lead electrocardiogram (ECG) data, has been developed. This breakthrough holds promise for future home-based prediabetes screening using consumer wearable devices, without requiring invasive blood tests.
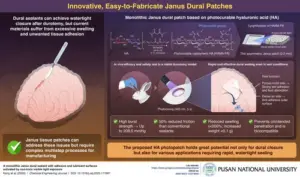
Pusan National University Researchers Develop Light-Activated Tissue Adhesive Patch for Rapid, Watertight Neurosurgical Sealing
Researchers develop an innovative dural patch that achieves rapid, watertight sealing of dural tears, boasting high biocompatibility

New method predicts asthma attacks up to five years in advance
Researchers at Mass General Brigham and Karolinska Institutet have identified a new method to predict asthma exacerbations with a high degree of accuracy.

‘Revoice’ device gives stroke patients their voice back
Researchers have developed a wearable, comfortable and washable device called Revoice that could help people regain the ability to communicate naturally and fluently following a stroke, without the need for invasive brain implants.

AI model predicts neural network degeneration patterns in ALS progression
New research from the University of St Andrews, the University of Copenhagen and Drexel University has developed AI computational models that predict the degeneration of neural networks in amyotrophic lateral sclerosis (ALS).
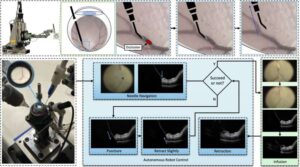
A new robotic system could perform delicate eye surgery
Retinal vein occlusion (RVO) is a severe disease that occurs when a vein in the light-sensitive layer at the back of the eye (i.e., the retina) becomes blocked, which results in a loss of vision. There are currently a few medical interventions that address RVO, including the periodic injection of medications that block the abnormal growth of blood vessels or of steroids, which reduce swelling and inflammation.

Autonomous AI agents developed to detect early signs of cognitive decline
A team of Mass General Brigham researchers has developed one of the first fully autonomous artificial intelligence (AI) systems capable of screening for cognitive impairment using routine clinical documentation.
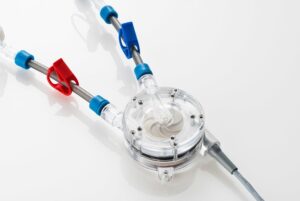
Amplifi Vascular wins FDA breakthrough nod for vein dilation system
Amplifi Vascular announced that it received breakthrough device designation for its Amplifi Vein Dilation System.
