MedTech News
.................... by Andrew Celentano
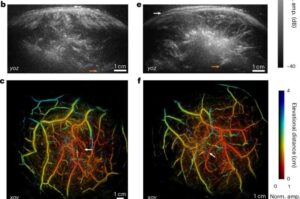
3D hybrid imaging system could address limitations of MRI, CT and ultrasound
In a proof-of-concept study, researchers from the Keck School of Medicine of USC and the California Institute of Technology (Caltech) have shown that an innovative, noninvasive technique can be used to quickly collect 3D images of the human body, from head to foot.
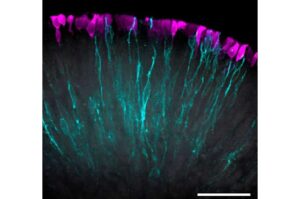
Vascularized retinal organoids engineered with functional light-signal pathways
Until now, it has been difficult to maintain retinal ganglion cells deep inside organoids over extended periods. The supply of nutrients and oxygen in the densely packed tissues is limited, leading to cell death.
Scientists discover natural ‘brake’ that could stop harmful inflammation
Researchers at University College London (UCL) have uncovered a key mechanism that helps the body switch off inflammation—a breakthrough that could lead to new treatments for chronic diseases affecting millions worldwide.

Alternative RNA splicing tied to schizophrenia-like behaviors in animal models
In a new study, Chinese researchers have discovered the previously unrecognized role of alternative splicing of the DOC2A gene in schizophrenia.

Johnson & Johnson MedTech launches mobile simulator for breast procedures
Johnson & Johnson (NYSE: JNJ)+
announced that it launched the Arbrea Breast Simulator Surgeon App for Mentor in the U.S.
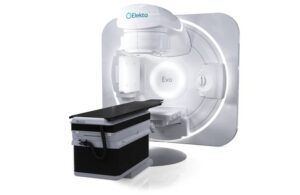
Elekta gets FDA nod for AI-powered linear accelerator for radiology
Elekta announced today that its Evo CT linear accelerator (linac) received FDA 510(k) clearance, making it available to U.S. radiation oncology professionals.
Finding new cell markers to track the most aggressive breast cancer in blood
Of all the types of breast cancer, triple negative breast cancer (TNBC) is the most aggressive and lacks specific therapies. TNBC is also more likely to metastasize, or travel through the bloodstream to spread to other organs, which causes most of breast cancer-related deaths each year.
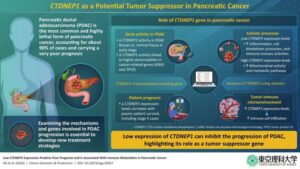
Potential tumor-suppressing gene identified in pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and begins in the cells lining the pancreatic duct. Accounting for more than 90% of all pancreatic cancers, PDAC is extremely difficult to treat and has a very high mortality rate.
