MedTech News
.................... by Andrew Celentano
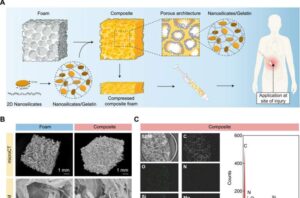
Stopping fatal blood loss with clay
Traumatic injury is the third leading cause of death in the state of Texas, surpassing strokes, Alzheimer’s disease and diabetes, according to the Centers for Disease Control and Prevention. A massive number of these deaths are the result of uncontrolled bleeding. “Severe blood loss can rapidly lead to hemorrhagic shock,” said Dr. Akhilesh Gaharwar, a biomedical engineering professor at Texas A&M University. “Many patients die within one to two hours of injury. This critical period is often referred to as the ‘golden hour.'”
Ancient mind-body practice proven to lower blood pressure in clinical trial
A traditional Chinese mind-body practice that combines slow, structured movement, deep breathing and meditative focus lowered blood pressure as effectively as brisk walking in a large randomized clinical trial published in JACC. Blood pressure reductions were seen after three months and sustained for one year.
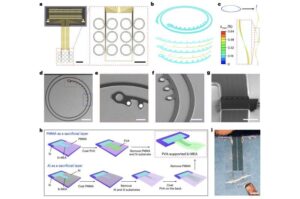
Kirigami-inspired sensors precisely map activity of neurons in the primate brain
Recent technological advances have opened new exciting possibilities for the development of smart prosthetics, such as artificial limbs, joints or organs that can replace injured, damaged or amputated body parts. These same advances are also enabling the development of other systems that connect the brain with machines, to record the activity of neurons or allow humans to operate machines in entirely new ways.
Medtronic gets FDA nod for Infuse bone graft in TLIF procedures
Medtronic (NYSE: MDT)+ announced today that it received FDA premarket approval (PMA) for the use of its Infuse bone graft in TLIF procedures.
LEX Diagnostics secures FDA clearance for VELO PCR system
The system is intended for use in urgent care clinics, primary care settings, physician office laboratories, and pharmacies.

Portal Diabetes Gets FDA Breakthrough for Pump, Starts Insulin Study
WESTFIELD, Ind., Feb. 17, 2026 /PRNewswire/ — Today, Portal Diabetes, Inc. (“Portal”) announced its receipt of the Breakthrough Device Designation by the Food and Drug Administration (FDA) for its implantable insulin pump system called “Portal Pump,” and the start of a Phase 1 study on its proprietary temperature-stable insulin (“Portal Insulin”).
Retia Medical Receives FDA 510(k) Clearance for Argos Infinity™, Expanding Cardiovascular Intelligence Across High-Risk Surgical and Critical Care Environments
WHITE PLAINS, N.Y., Feb. 17, 2026 /PRNewswire/ — Retia Medical today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Argos Infinity™, the company’s cardiovascular intelligence software platform designed for high-risk surgical and critical care environments across health systems.

FDA 510(k) Clearance Establishes Broad Intended Use for Copan’s PhenoMATRIX®, Expanding Clinical Microbiology Capabilities
MURRIETA, Calif., Feb. 17, 2026 /PRNewswire/ — Copan Group announced today that PhenoMATRIX®, its automated image assessment software used with WASPLab® full laboratory automation, received FDA 510(k) clearance as a Class II device in the United States.
