MedTech News
.................... by Andrew Celentano
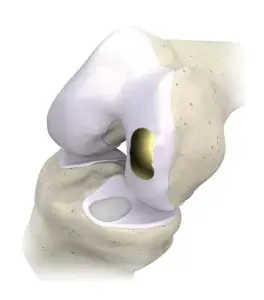
Overture Orthopaedics Announces Milestone $1.0Mn in Sales of its OvertureTi Knee Resurfacing System
OvertureTi Knee Resurfacing System implants are designed specifically as an alternative when it is still too early for arthroplasty
KORU Medical seeks FDA clearance for infusion system
KORU Medical has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) seeking approval for its FreedomEDGE infusion system.

A protein that makes hydrogen sulfide shows potential as a therapeutic target for Alzheimer’s disease
Scientists at Johns Hopkins Medicine say results of a new study are advancing efforts to exploit a new target for Alzheimer’s disease: a protein that manufactures an important gas in the brain.

Medtech M&A: The biggest deals of 2025
In 2025, the medtech industry saw another year full of all kinds of deals in the mergers and acquisitions (M&A) arena.

The top diabetes tech stories of 2025
As the year comes to a close, it’s time to look back at the numerous innovations across the diabetes technology industry in 2025.

AI-powered knowledge graph links heart images to genes and drug predictions
CardioKG provides a detailed view of the heart’s structure and function which dramatically improves the accuracy of predicting which genes are linked to disease and whether existing drugs could treat them.
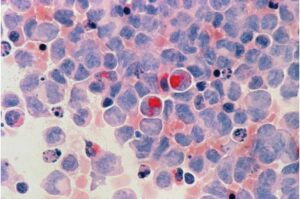
Breast cancer drug boosts leukemia treatment: Unexpected duo shows promise in overcoming resistance
A research team at Oregon Health & Science University has discovered a promising new drug combination that may help people with acute myeloid leukemia overcome resistance to one of the most common frontline therapies.
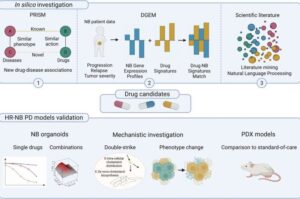
Machine learning identifies statin and phenothiazine combo for neuroblastoma treatment
Researchers at Lund University in Sweden have identified a combination of statins and phenothiazines that is particularly promising in the treatment of the aggressive form of neuroblastoma.
