MedTech News
.................... by Andrew Celentano
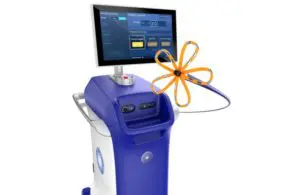
Boston Scientific wins expanded CE mark for Farapulse in persistent AFib
Boston Scientific (NYSE: BSX)+ announced today that its Farapulse pulsed field ablation (PFA) system received expanded CE mark approval.

BD earns FDA 510(k) clearance for Surgiphor 1000mL system
BD (NYSE: BDX)+ received FDA 510(k) clearance for its Surgiphor 1000mL antimicrobial irrigation system, the company announced today.

Senseonics earns FDA IDE for next-gen Gemini CGM, posts Q4 revenue beat
Senseonics (NYSE:SENS) posted fourth-quarter results that proved mixed compared to Wall Street forecasts alongside a major pipeline announcement.
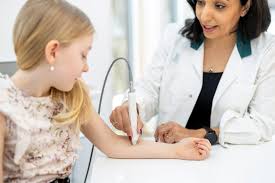
SciBase receives approval by FDA for extended labelling in the US
STOCKHOLM, March 2, 2026 /PRNewswire/ — SciBase Holding AB (“SciBase”) (STO: SCIB), a leading developer of AI-based diagnostic solutions for skin disorders, today announced that it has received approval by FDA for its supplement to extend the labelling to include other healthcare professionals and not only dermatologists to perform the Nevisense procedure

Ultrasound AI Receives FDA De Novo Clearance for Delivery Date AI Technology
DENVER, March 2, 2026 /PRNewswire/ — Ultrasound AI, a pioneer in artificial intelligence applications for medical imaging, today announced it has received FDA De Novo clearance for its flagship Delivery Date AI technology, a cloud-based SaMD that determines a Predicted Delivery Date (PDD) solely from standard ultrasound images and seamless integration into current OB/MFM prenatal visit workflows; PDD is provided in real-time for actionable decision-making by the clinical team.

New record: Laser for surgery cuts bone deeper than before
Lasers cut precisely and without contact—ideal for surgery. The problem is that in hard tissues such as bone, they are too slow and do not cut deep enough. Researchers at the University of Basel have now demonstrated a way to cut much deeper and faster with a surgical laser than with previous laser systems.

Synergy Spine Solutions® Receives FDA Approval for its Synergy Disc®, Expanding Cervical Disc Replacement Options for U.S. Patients
Synergy Spine Solutions®, a medical device company focused on improving the quality of life for patients undergoing spine surgery, today announced it has received U.S. Food and Drug Administration (FDA) Premarket Approval (PMA) for the Synergy Disc® for 1-level indications at C3-C7.

Engineered protein markers read living brain gene activity in monkeys via blood
Gene therapy has been successfully used to treat a number of diseases, including immune deficiencies, hereditary blindness, hemophilia and, recently, Huntington’s disease, a fatal neurological disorder.
