MedTech News
.................... by Andrew Celentano
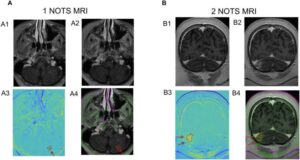
Focused ultrasound passes first test in treatment of pediatric brain cancer
Columbia University researchers are the first to show that focused ultrasound—a noninvasive technique that uses sound waves to enhance the delivery of drugs into the brain—can be safely used in children being treated for brain cancer.

Vision can be rebooted in adults with amblyopia, study suggests
Temporarily anesthetizing the retina briefly reverts the activity of the visual system to that observed in early development and enables growth of responses to the amblyopic eye, new research shows.
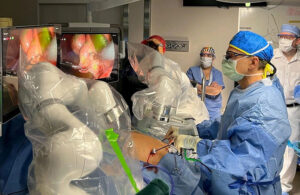
FDA clears Levita’s Magnetic Surgical System for pediatric use; Cleveland Clinic Children’s performs first US surgery
Levita Magnetics has received FDA 510(k) clearance for its Magnetic Surgical System (MSS) to be used in certain pediatric surgeries, with the first U.S. surgery performed at Cleveland Clinic Children’s earlier this month.

FDA clears Onward’s ARC-EX System for home use in spinal cord injury care
Having been commercially available for less than 12 months, the system is now offered in over 60 US clinics.

Carea launches new Postpartum Mum Tracker
Carea, a pregnancy and postnatal wellbeing app that prioritises women’s health, has launched the Postpartum Mum Tracker to support the 1 in 5 new mothers who face post-birth mental health struggles.

Vesalio Receives Two FDA 510(k) Clearances, Advancing Its Comprehensive Thrombectomy Platform with Aspiration Technology
PLANO, Texas, Nov. 18, 2025 /PRNewswire/ — Vesalio, a leader in innovative thrombectomy solutions, today announced two new FDA 510(k) clearances for its aspiration devices, designed for peripheral and neurovascular applications. These clearances mark a key milestone in Vesalio’s evolution toward providing a complete suite of thrombectomy products across multiple vascular territories.

Nanopath Granted FDA Breakthrough Device Designation for Its Molecular Diagnostic Platform for Urinary Tract Infections
CAMBRIDGE, Mass., Nov. 18, 2025 /PRNewswire/ — Nanopath, a point-of-care diagnostics company enabling high-quality molecular testing in minutes, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation for the company’s novel assay for the rapid detection of infection in patients with suspected, or at risk of, complicated urinary tract infections (UTIs).
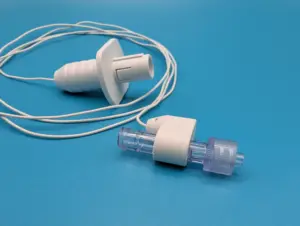
Piccolo Medical® Receives FDA Clearance to Expand ECGuide™ Technology Use to Pediatric and Neonatal Patients
SAN FRANCISCO, Nov. 18, 2025 /PRNewswire/ — Piccolo Medical, Inc. (Piccolo) today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to expand the Indications for Use for its PM2™ System and ECGuide™ Connector. This clearance extends the technology’s use as an alternative to chest x-ray for a variety of central venous access devices in pediatric and neonatal patient populations.
