MedTech News
.................... by Andrew Celentano
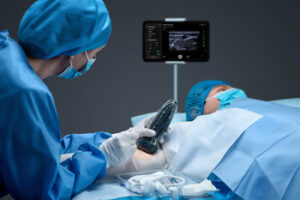
Mindray Introduces TE Air e5M Wireless Handheld Ultrasound to Revolutionize Whole-Body Scanning
MAHWAH, N.J., Nov. 19, 2025 /PRNewswire/ — Mindray, a leading global developer of healthcare technologies and solutions specializing in patient monitoring, anesthesia, and ultrasound, continues to push the boundaries of innovation with its cutting-edge, whole-body wireless scanning system, the TE Air e5M Ultrasound Machine.

Flexible organic electrodes convert infrared light into nerve signals in damaged retinas
In an important step toward visual prostheses, biocompatible electrodes can convert infrared light into nerve impulses, as demonstrated by a team at TU Wien
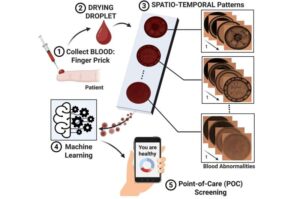
Researchers diagnose disease with a drop of blood, a microscope and AI
Not long ago, the idea of diagnosing a disease with a droplet of blood was considered a pipe dream. Today, this technology could soon become a reality.

3D-printed blood vessels could unravel secrets of strokes
3D printed blood vessels on glass that mimic blood vessel anatomy and the fluid dynamics of blood flow could be an invaluable tool in studying the causes of stroke, new research from a University of Sydney team has found and it has already led to important insights.
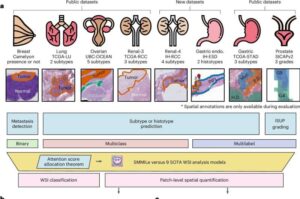
AI tool can analyze complex cancer images rapidly—offering potential to personalize treatment
Complex digital images of tissue samples that can take an experienced pathologist up to 20 minutes to annotate could be analyzed in just one minute using a new AI tool developed by researchers at the University of Cambridge.
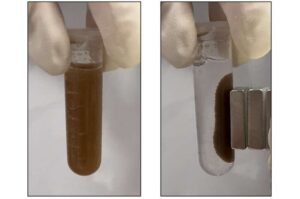
Bacteria ‘pills’ could detect gut diseases—without the endoscope
Colonoscopies may one day have some competition—researchers report in ACS Sensors that they’ve developed a sensor made of tiny microspheres packed with blood-sensing bacteria that detect markers of gastrointestinal disease. Taken orally, the miniature “pills” also contain magnetic particles that make them easy to collect from stool.
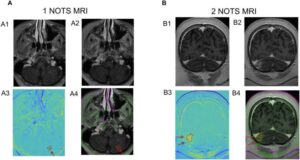
Focused ultrasound passes first test in treatment of pediatric brain cancer
Columbia University researchers are the first to show that focused ultrasound—a noninvasive technique that uses sound waves to enhance the delivery of drugs into the brain—can be safely used in children being treated for brain cancer.

Vision can be rebooted in adults with amblyopia, study suggests
Temporarily anesthetizing the retina briefly reverts the activity of the visual system to that observed in early development and enables growth of responses to the amblyopic eye, new research shows.
