MedTech News
.................... by Andrew Celentano
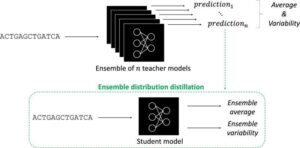
AI tool debuts with better genomic predictions and explanations
Artificial intelligence has taken the world by storm. In biology, AI tools called deep neural networks (DNNs) have proven invaluable for predicting the results of genomic experiments. Their usefulness has these tools poised to set the stage for efficient, AI-guided research and potentially lifesaving discoveries—if scientists can work out the kinks. The findings are published in the journal npj Artificial Intelligence.
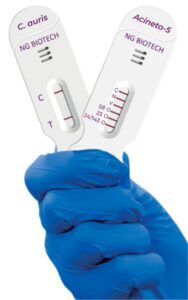
NG Biotech announces FDA approval for diagnostic assays
NG Biotech manufactured the assays in France, with Hardy Diagnostics serving as the exclusive distributor in the US.

FDA expands age indication for Staar Surgical EVO ICL
Staar Surgical (Nasdaq:STAA) announced that it received FDA approval for an expanded age indication for its Evo lenses.

Scientist invents super-chipped shoe to help his 89-year-old mentor avoid falling
When a big-hearted engineer noticed his 89-year-old mentor was unsteady on his feet, he sprang into action and created a futuristic shoe that could in the future help him—and scores of other older people—keep their balance.
Machine-learned biomarker identifies those at high risk for liver cancer
Researchers led by Xian-Yang Qin at the RIKEN Center for Integrative Medical Sciences (IMS) in Japan have developed a score that predicts the risk of liver cancer. Published in the journal Proceedings of the National Academy of Sciences, the study establishes that the protein MYCN drives liver tumorigenesis, specifically of the type of tumors found in the deadliest subtype of liver cancer.

Pop-up-style 3D electrode array captures organoid-wide brain rhythms in real time
A team led by Northwestern University and Shirley Ryan AbilityLab scientists have developed a new technology that can eavesdrop on the hidden electrical dialogues unfolding inside miniature, lab-grown human brain-like tissues.
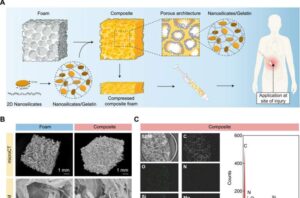
Stopping fatal blood loss with clay
Traumatic injury is the third leading cause of death in the state of Texas, surpassing strokes, Alzheimer’s disease and diabetes, according to the Centers for Disease Control and Prevention. A massive number of these deaths are the result of uncontrolled bleeding. “Severe blood loss can rapidly lead to hemorrhagic shock,” said Dr. Akhilesh Gaharwar, a biomedical engineering professor at Texas A&M University. “Many patients die within one to two hours of injury. This critical period is often referred to as the ‘golden hour.'”
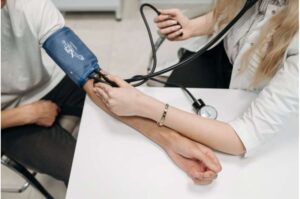
Ancient mind-body practice proven to lower blood pressure in clinical trial
A traditional Chinese mind-body practice that combines slow, structured movement, deep breathing and meditative focus lowered blood pressure as effectively as brisk walking in a large randomized clinical trial published in JACC. Blood pressure reductions were seen after three months and sustained for one year.
