MedTech News
.................... by Andrew Celentano

Magnetic field therapy shows promise in mimicking exercise benefits for type 2 diabetes patients with central obesity
Researchers from Singapore General Hospital (SGH) and National University of Singapore (NUS) found that using pulsed electromagnetic fields to stimulate muscle tissue and mimic the effects of exercise could benefit patients with type 2 diabetes with excess belly fat.
FDA grants 510(k) clearance to Sirona Medical’s imaging suite
The clearance from the US regulator broadens the company’s diagnostic imaging offerings.
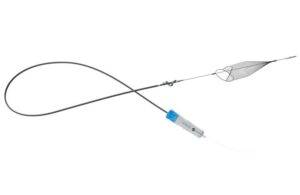
InterVene wins FDA 510(k) for Recana thrombectomy catheter
InterVene announced today that it received FDA 510(k) clearance for its Recana thrombectomy catheter system.
Roche secures CE mark for acute dengue virus infection diagnostic test
Studies showed that the assay demonstrated a 94.90% sensitivity in polymerase chain reaction-confirmed positive samples.

Could Fat-Trapping Boba Pearls Replace Weight Loss Medications Like GLP-1 Agonists?
Learn how microbeads made from green tea polyphenols, vitamin E, and seaweed could help break down fats without the negative side effects that can come from GLP-1 agonists.

Tosoh Bioscience Receives FDA 510(k) Clearance for Fast, Accurate GR01 HbA1c Testing Analyzer
GROVE CITY, Ohio, Oct. 30, 2025 /PRNewswire/ — Tosoh Bioscience, Inc., a market leader in clinical diagnostics has received U.S. FDA 510(k) clearance for its next-generation Tosoh Automated Glycohemoglobin Analyzer HLC®-723 GR01 (GR01) for HbA1c testing.
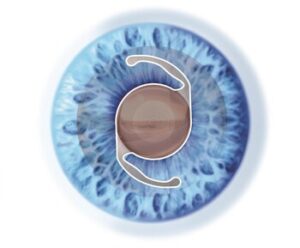
Revolutionary Cataract Surgery Technology Transforms Eye Care with Precision and Enhanced Patient Outcomes
SCOTTSDALE, Ariz., Oct. 30, 2025 /PRNewswire/ — In an exciting breakthrough for eye health, Barnet Dulaney Perkins, a leader in ophthalmic innovation, uses an advanced cataract surgery technique that delivers faster recovery, increased precision, and exceptional long-term results. The approach, which incorporates cutting-edge technology, revolutionizes how cataracts are treated, benefiting millions of patients worldwide.

Laser eye treatment shows potential for halting dry macular degeneration progression in animal models
Around a third of people over the age of 80 suffer from age-related macular degeneration (AMD), with an estimated 20 million Americans aged 40 and older currently living with AMD.
