MedTech News
.................... by Andrew Celentano

First-in-human trial of CRISPR gene-editing therapy safely lowers cholesterol and triglyceride
“This is really unprecedented. A single treatment that simultaneously lowered LDL cholesterol and triglycerides,” said Luke J. Laffin, M.D., lead study author and a preventive cardiologist at the Cleveland Clinic

Tailored heart pump could transform care for half of heart failure patients
A Monash University study proposes an innovative heart pump design could address the unique challenges of this condition by improving blood flow and alleviating the strain on the heart.

Zero-cost, AI-driven digital detection identifies Alzheimer’s without additional clinician time
Few primary care practices are designed for the timely detection of Alzheimer’s disease and related dementias. The limited time that primary care clinicians are able to spend with patients, the need to focus on the health problems that brought the patient to the clinic, as well as the stigma of Alzheimer’s disease and dementia are major reasons for lack of recognition of the condition.
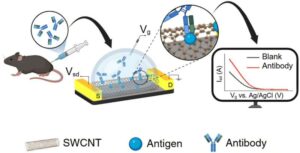
No-needle test can tell if flu/COVID vaccines are effective
A team of researchers at the University of Pittsburgh has developed a skin patch that can detect antibodies associated with COVID and flu infections. It’s orders of magnitude more sensitive than existing tests, uses just a half volt of electricity, and can return results in 10 minutes.
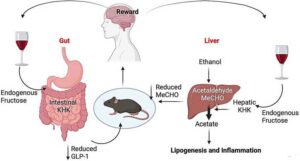
One enzyme could be behind alcohol addiction and liver disease
Scientists have uncovered a surprising connection between sugar metabolism and alcohol addiction, identifying a potential new therapeutic target for treating alcohol-associated liver disease (ALD) and alcohol use disorder (AUD).

Protein linked to cancer found to play key role in wound healing
When doctors detect elevated levels of SerpinB3 in a blood test, it can signal that something is seriously wrong, from hard-to-treat cancers to severe inflammatory conditions.
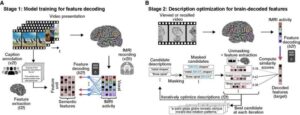
‘Mind-captioning’ technique can read human thoughts from brain scans
Reading brain activity with advanced technologies is not a new concept. Generating detailed, structured descriptions of complex visual perceptions or thoughts remains difficult.

TAICEND’s Patented Fish Collagen Technology Brings a Breakthrough in the Healing of Hard-to-Heal Wounds
KAOHSIUNG CITY, Taiwan, Nov. 7, 2025 /PRNewswire/ — TAICEND is set to officially launch its groundbreaking new product — TAICEND Collagen Dressing — in 2026. Extracted from premium fish skin, the collagen is produced through a patented low-temperature extraction technology combined with a proprietary formulation, resulting in a high-purity fish collagen suitable for advanced wound dressings.
