
Axonics wins CE mark for 4th-gen rechargeable sacral neuromod system
Axonics (Nasdaq:AXNX) announced today that it received CE mark approval for its R20 rechargeable sacral neuromodulation (SNM) system.

Axonics (Nasdaq:AXNX) announced today that it received CE mark approval for its R20 rechargeable sacral neuromodulation (SNM) system.

SafeGuard Surgical announced today that the FDA granted breakthrough device designation to its LeakGuard biodegradable stent.
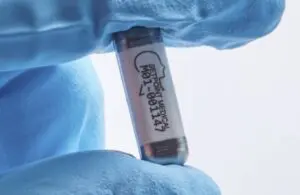
SetPoint Medical announced today that received FDA breakthrough device designation for its novel neuroimmune modulation platform.

The collaborators aim to resolve the clinical, regulatory, coverage and payment questions facing developers of the devices.
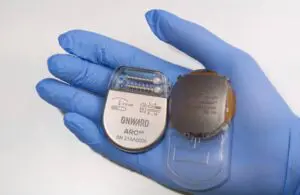
Onward Medical announced today that the FDA accepted it into its new Total Product Lifecycle Advisory Program (TAP) — a move that could boost the development of Onward’s BCI technology.

Pulse Biosciences (Nasdaq:PLSE) announced that the FDA granted 510(k) clearance for its CellFX nsPFA percutaneous electrode system.
One panelist who voted yes said the “incremental benefits outweigh the small risks of anaphylaxis.”

Getinge this week announced a major FDA clearance and the beginning of a U.S. launch of multiple surgical tools.
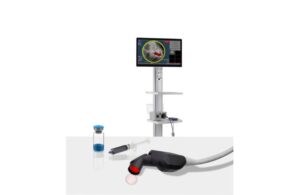
Lumicell announced that an FDA advisory committee voted in support of the benefit-risk profile of its LumiSight offering.

Beckman Coulter Life Sciences, a laboratory automation and innovation company, has received 510(k) clearance from the Food and Drug Administration (FDA) to distribute its DxFLEX Clinical Flow Cytometer in the United States.