MedTech News
.................... by Andrew Celentano

AI-built maps reveal causal gene regulation across Alzheimer’s brain cell types
Researchers led by Min Zhang and Dabao Zhang of the University of California, Irvine’s Joe C. Wen School of Population & Public Health have created the most detailed maps to date showing how genes causally regulate one another across different types of brain cells affected by Alzheimer’s disease.
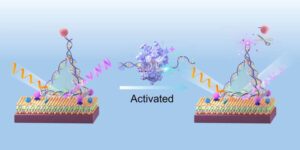
Light-based sensor detects early molecular signs of cancer in the blood
Researchers have developed a highly sensitive light-based sensor that can detect extremely low concentrations of cancer biomarkers in the blood.
Unraveling the mystery of why some cancer treatments stop working
Cancer researchers working on immunotherapies have made a big discovery: SLAMF6, a molecule on the surface of immune cells that prevents T cells from effectively attacking tumors—and, in mice, they’ve found a way to neutralize it.
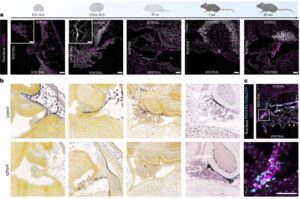
Scientists discover new gatekeeper cell in the brain
Researchers have identified and characterized a previously unknown cellular barrier in the brain, which sheds new light on how the brain is protected from the rest of the body.

Common anti-seizure drug prevents Alzheimer’s plaques from forming
While other drugs clear existing plaques, levetiracetam prevents production of toxic amyloid beta peptides

Using synthetic biology and AI to address global antimicrobial resistance threat
Driven by overuse and misuse of antibiotics, drug-resistant infections are on the rise, while development of new antibacterial tools has slowed.
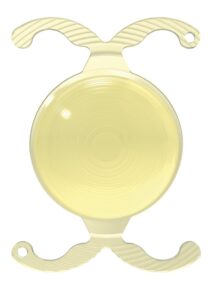
BVI completes first U.S. implants of trifocal IOL
BVI announced the first successful U.S. implantations of the FDA-approved FineVision HP hydrophobic trifocal intraocular lens (IOL).

Labcorp Launches First FDA-Cleared Blood Test for Alzheimer’s Disease Assessment in Primary Care
BURLINGTON, N.C., Feb. 11, 2026 /PRNewswire/ — Labcorp (NYSE: LH), a global leader of innovative and comprehensive laboratory services, announced today the nationwide availability of the Elecsys® pTau-181 test, the first and only blood test cleared by the U.S. Food and Drug Administration (FDA) to aid in the initial assessment of Alzheimer’s disease in the primary care setting. This launch further expands Labcorp’s comprehensive portfolio of Alzheimer’s disease blood tests, offering clinicians solutions across both primary and specialty care settings.
