MedTech News
.................... by Andrew Celentano
New biosensor technology could improve glucose monitoring
A wearable biosensor developed by Washington State University researchers could improve wireless glucose monitoring for people with diabetes, making it more cost-effective, accurate, and less invasive than current models.
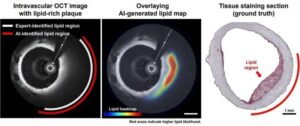
Combining AI with optical coherence tomography shows potential for detecting lipid-rich plaques in coronary arteries
Researchers have developed a new artificial intelligence-based approach for detecting fatty deposits inside coronary arteries using optical coherence tomography (OCT) images.
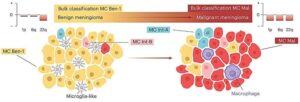
Doctors discover a simple method to predict the risk of brain tumor recurrence
“By using a simple and inexpensive technique that pathologists already use every day, it is now possible to make a better risk assessment, even in countries where advanced technologies are not available.”
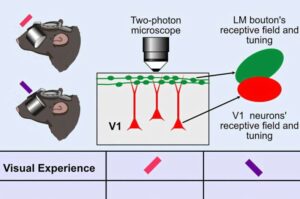
Mice with miniature goggles reveal how different visual experiences give rise to different neural wiring
Rresearchers reared mice fitted with miniature goggles that biased their perception of the visual world. One group of animals only saw edges oriented at a certain angle, while the other saw edges oriented at a different angle.

Wearable trackers can detect depression relapse weeks before it returns, study finds
New research from McMaster University suggests that disruptions in a person’s sleep and daily activity routine, as detected through a simple wrist-worn device, can signal when there is increased risk of relapsing into major depression.
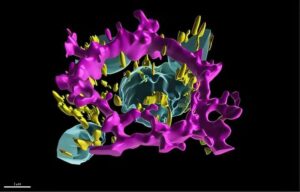
Digital twin reveals how eye cells lose their organization in leading cause of vision loss
National Institutes of Health (NIH) researchers have developed a digital replica of crucial eye cells, providing a new tool for studying how the cells organize themselves when they are healthy and affected by diseases.

Nearly three quarters of US baby foods are ultra-processed, new study finds
An alarming 71% of grocery store baby food products in the United States are classified as ultra-processed foods (UPFs), according to new research published in the journal Nutrients.
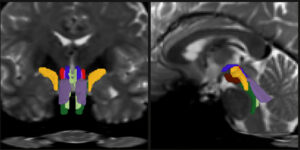
AI algorithm enables tracking of vital white matter pathways
Opening a new window on the brainstem, a new tool reliably and finely resolves distinct nerve bundles in live diffusion MRI scans, revealing signs of injury or disease.
