MedTech News
.................... by Andrew Celentano
Noninvasive brain scanning could send signals to paralyzed limbs
People with spinal cord injuries often lose some or all their limb function. In most patients, the nerves in their limbs work fine, and the neurons in their brain are still operational, but the damage to their spinal cords prevents the two areas from communicating.

Illumina secures CMS reimbursement for TruSight™ Oncology Comprehensive, expanding access to precision oncology
The FDA-approved comprehensive genomic profiling test will be reimbursed at a rate of $2,989.55 per test, helping to advance adoption in the US healthcare system
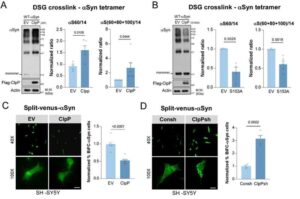
Potential new target to treat Parkinson’s disease discovered
About 1 million Americans suffer from Parkinson’s disease, with around 90,000 new cases diagnosed each year, according to the Parkinson’s Foundation. The chronic, degenerative brain disorder destroys dopamine-producing cells essential for smooth, coordinated movement.
Abbott picks up CE mark for TactiFlex dual-energy ablation catheter
Abbott (NYSE: ABT)+
announced today that it received CE mark for its TactiFlex Duo Ablation Catheter, Sensor-Enabled, to treat AFib.
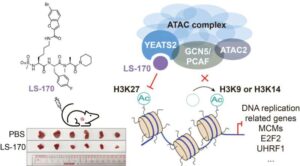
A new strategy to beat lung cancer: Chemists develop first-in-class inhibitor targeting a key epigenetic regulator
A research team has made a breakthrough in epigenetic drug discovery. The researchers have successfully developed a first-in-class chemical inhibitor that precisely and selectively targets the ATAC complex, a critical cellular “switch operator” that activates tumor-promoting genes, opening a novel therapeutic avenue for non-small cell lung cancer (NSCLC).

Gut microbiota can predict long-term complications of acute pancreatitis
A Europe-wide study led by the University Medical Center Göttingen (UMG) shows that the microbial composition of the gut, known as the gut microbiome, can predict long-term complications following severe acute pancreatitis.
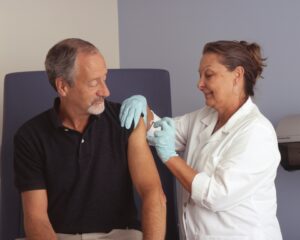
Shingles vaccine linked to slower biological aging in older adults
Shingles vaccination not only protects against the disease but may also contribute to slower biological aging in older adults, according to a new USC Leonard Davis School of Gerontology study.

Blood test can identify cancer in patients with non-specific symptoms
A simple blood test can help detect cancer in patients with non-specific symptoms such as fatigue, pain or weight loss. This is according to a Swedish study from Karolinska Institutet, Danderyd Hospital and others, published in Nature Communications.
