MedTech News
.................... by Andrew Celentano
New blood test signals who is most likely to live longer, study finds
As people age, it becomes harder to know who is on track for healthy years ahead and who may be at higher risk for serious decline. A new study suggests that part of the answer may already be circulating in the bloodstream.
Cancer‑Eating Bacteria Could Devour Tumors From the Inside Out
Learn how reseachers are working to engineer certain bacteria to consume tumors.
Medtronic launches MiniMed Go smart MDI system with Simplera sensor in Europe
Medtronic (NYSE:MDT) announced today that it launched its MiniMed Go smart multiple daily injection (MDI) system with the Simplera sensor.
Carea launches support platform for complex fertility treatment
Carea, a pregnancy and postnatal wellbeing app, is bridging this gap with the launch of its new Trying to Conceive: IVF/IUI Mode, to support the tens of thousands of women undergoing complex fertility treatment, amid growing concern over inconsistent clinic continuity and limited day-to-day guidance.
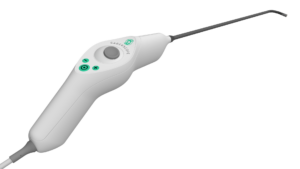
Xenocor Announces FDA Clearance of Saberscope®, the First Single-Use 5mm Articulating Laparoscope for Enhanced Surgical Visualization
SALT LAKE CITY, Feb. 24, 2026 /PRNewswire/ — Xenocor, Inc. today announced that the U.S. Food and Drug Administration (FDA) has cleared the new Xenocor Saberscope®, a single-use 5mm articulating laparoscope designed for high definition (HD) visualization during minimally invasive abdominal (belly) and thoracic (chest) surgical procedures.

AEYE Health’s AI Retinal Imaging Redefines Non-Invasive Blood Pressure Monitoring
NEW YORK, Feb. 24, 2026 /PRNewswire/ — AEYE Health, the leader in ophthalmic AI and retinal diagnostics and the developer of AEYE-DS, the fastest growing solution for diabetic eye exams in the U.S., today announced the publication of a landmark study in BMJ Innovations.

A human mini-bladder shows the culprit of recurrent infections
Researchers at EPFL, Heidelberg University and Roche have built a human mini-bladder to show how urine composition weakens bladder tissue, helping infections recur even after antibiotics. The work was led by John McKinney (EPFL) Matthias Lütolf (Roche Institute of Human Biology/EPFL), and Vivek Thacker (Heidelberg University) and is now published in Nature Communications.
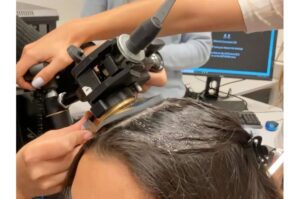
Ultrasound gives the brain a nudge in the right direction
Neuroscientist Soha Farboud of the Donders Institute at Radboud University has succeeded in adjusting activity in specific brain areas using a new technique. With ultrasonic brain stimulation, she was able to influence whether people chose to look left or right. A key advantage of ultrasound is that, unlike existing methods, it can safely reach deep brain regions from outside the skull.
