MedTech News
.................... by Andrew Celentano
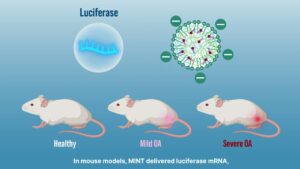
Research Spotlight: Developing “Smart” Nanoparticles to Deliver Targeted Gene Therapy in Osteoarthritis
Nanoparticles are engineered to home in on areas where cartilage has degenerated in osteoarthritis, ensuring that treatment concentrates exactly where it is needed
IotaMotion wins expanded FDA nod for pediatric use of robotic-assisted cochlear implant placement
IotaMotion announced today that it received FDA 510(k) clearance for expanded pediatric use of its iotaSoft insertion system.

Boston Scientific wins CE mark for Embold detachable coil system
Boston Scientific (NYSE: BSX)+
announced that it received CE mark approval for its Embold detachable coil system.
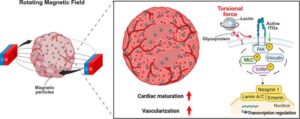
Three-dimensional magnetic torque enables heart mechanics in organoids
Heart disease remains the leading cause of death worldwide, yet progress in understanding and treating cardiac disorders is limited by the shortcomings of existing experimental models. Traditional animal models often fail to capture human-specific cardiac biology, while conventional two-dimensional cell cultures lack the functional and structural complexity of heart tissue.

Simple tool predicts who is most at risk of dementia after stroke
A new international study led by researchers from UNSW Sydney’s Center for Healthy Brain Aging (CHeBA) has developed the first practical, five-year dementia risk prediction tool for stroke survivors—using only information that’s routinely collected in hospitals and clinics.

Tiny sensor could transform head injury detection
A tiny sensor that detects hazardous head impacts the instant they occur could reshape safety monitoring in sports, transportation and other high-risk settings.

When a virus releases the immune brake: New evidence on the onset of multiple sclerosis
Autoimmune diseases such as multiple sclerosis arise when the immune system turns against the body itself. Yet for most of them, it remains unclear why this process begins. Researchers have now identified how the Epstein-Barr virus can, under specific conditions, initiate early multiple sclerosis-like damage in the brain. This offers a new perspective on how rare immune events may shape disease risk.

Sequel Med Tech, Diabeloop to integrate automated insulin delivery algorithm into twiist pump
Sequel Med Tech and Diabeloop today announced a collaboration to integrate a new algorithm into the twiist automated insulin pump.
