MedTech News
.................... by Andrew Celentano

Nanopath Granted FDA Breakthrough Device Designation for Its Molecular Diagnostic Platform for Urinary Tract Infections
CAMBRIDGE, Mass., Nov. 18, 2025 /PRNewswire/ — Nanopath, a point-of-care diagnostics company enabling high-quality molecular testing in minutes, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation for the company’s novel assay for the rapid detection of infection in patients with suspected, or at risk of, complicated urinary tract infections (UTIs).
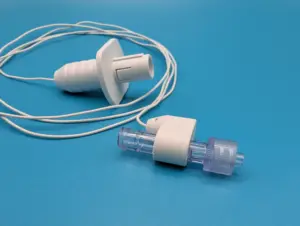
Piccolo Medical® Receives FDA Clearance to Expand ECGuide™ Technology Use to Pediatric and Neonatal Patients
SAN FRANCISCO, Nov. 18, 2025 /PRNewswire/ — Piccolo Medical, Inc. (Piccolo) today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to expand the Indications for Use for its PM2™ System and ECGuide™ Connector. This clearance extends the technology’s use as an alternative to chest x-ray for a variety of central venous access devices in pediatric and neonatal patient populations.
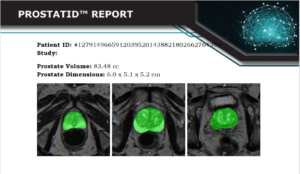
Bot Image, Inc. Earns UKCA Certification for ProstatID® AI Prostate MRI Software Under EU MDR 2002 No. 618
OMAHA, Neb., Nov. 18, 2025 /PRNewswire/ — Bot Image, Inc., a leader in AI-powered diagnostic imaging, today announced that its flagship software, ProstatID®, has achieved UKCA certification under the European Union’s Medical Device Regulation (EU) 2017/745, marking yet another major milestone in the company’s global expansion.
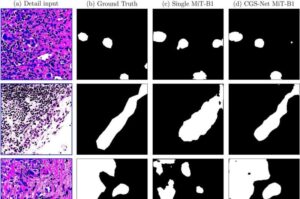
AI tool mimics pathologists to improve breast cancer tissue analysis accuracy
A research team led by two University of Maine Ph.D. students developed an artificial intelligence (AI) system that could make it easier and faster for doctors to identify signs of breast cancer in tissue samples, possibly preventing delays and saving lives.
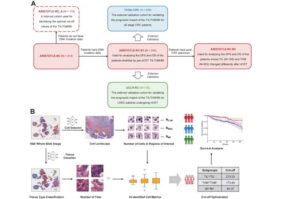
AI-assisted immune profiling helps predict treatment success in rectal cancer patients
Artificial intelligence (AI) can predict how well patients with rectal cancer will respond to treatment by analyzing standard tissue samples taken during diagnosis, finds a new study from researchers at UCL and UCLH.
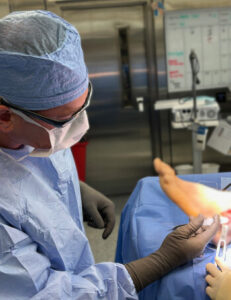
Alafair Biosciences Announces First Clinical Use of VersaCoat™ Flowable Hydrogel in Foot & Ankle and Hand & Wrist Procedures
AUSTIN, Texas, Nov. 18, 2025 /PRNewswire/ — Alafair Biosciences, Inc., a medical device company redefining soft-tissue protection in orthopedic surgery, announced today the first clinical use of its VersaCoat™ Flowable Hydrogel (VersaCoat™ Tendon Protector, VersaCoat™ Nerve Protector) by two leading surgeons in lower and upper extremity procedures.

Using robotic testing to spot overlooked sensory deficits in stroke survivors
A decade ago, at age 55, Don Lewis suffered a stroke in his sleep. When he woke up, he couldn’t move his left arm or leg. Lewis’s neighbor realized his truck hadn’t moved in two days and called 911 for a welfare check. When paramedics found him, he was paralyzed on one side.
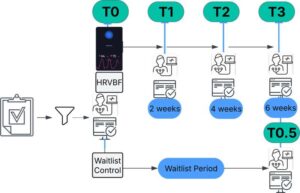
Heart rate monitor can improve PTSD and chronic pain symptoms
A new study led by researchers from Murdoch University’s School of Psychology, Personalized Medicine Center, and Center for Healthy Aging, Health Futures Institute has found that heart rate variability biofeedback can significantly reduce symptoms of both PTSD and chronic pain—two conditions that frequently co-occur and are notoriously difficult to treat together.
