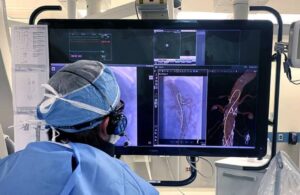
FDA approves enhanced Philips LumiGuide endovascular navigation wire
Philips (NYSE: PHG)+
announced today that it introduced the FDA-approved, 160 cm version of its LumiGuide endovascular navigation wire.

Philips (NYSE: PHG)+
announced today that it introduced the FDA-approved, 160 cm version of its LumiGuide endovascular navigation wire.

Commercial partner Ascensia is in discussions with insulin pump manufacturers to create an automated insulin delivery system.

Biotronik announced today that it received FDA approval for its Selectra 3D catheter and Solia S lead for use in left bundle branch area pacing (LBBAP).
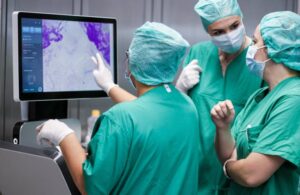
SamanTree Medical announced today that it received FDA 510(k) clearance for its Histolog scanner for tissue imaging.

SILVER SPRING, Md., Sept. 12, 2024 /PRNewswire/ — Today, the U.S. Food and Drug Administration authorized the first over-the-counter (OTC) hearing aid software device, Hearing Aid Feature, intended to be used with compatible versions of the Apple AirPods Pro headphones. Once installed and customized to the user’s hearing needs, the Hearing Aid Feature enables compatible versions of the AirPods Pro to serve as an OTC hearing aid, intended to amplify sounds for individuals 18 years or older with perceived mild to moderate hearing impairment.

Hinge Health announced that it launched its Enso 3 FDA-cleared wireless device for reducing musculoskeletal pain.

BETHLEHEM, Pa., Sept. 10, 2024 /PRNewswire/ — Tyber Medical LLC, a leading orthopedic device manufacturer specializing in private label implants for the extremity, trauma, and spine markets, is proud to announce that its PEEK ToeGrip Hammer Toe implant family has received Medical Device Regulation (MDR) certification from TÜV Rheinland. This prestigious certification marks the first time a hammer toe implant has achieved both FDA 510(k) clearance and MDR certification, setting a new standard for orthopedic implants.
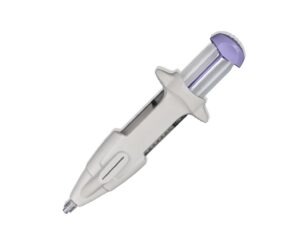
Femasys today announced it received FDA 510(k) clearance for its FemChec contrast-generating device that checks fallopian tubal status.
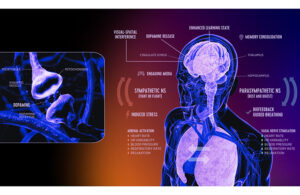
DeepWell Digital Therapeutics (DTx) has won FDA 510(k) clearance of technology to be used in immersive media like video games for stress reduction and as an adjunctive treatment for high blood pressure.

CENTER VALLEY, Pa., Sept. 5, 2024 /PRNewswire/ — Odin Medical Ltd., an Olympus Corporation company, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the first cloud-based Artificial Intelligence (AI) technology designed to assist gastroenterologists in detecting suspected colorectal polyps during colonoscopy procedures, the CADDIE™ computer-aided detection (CADe) device.