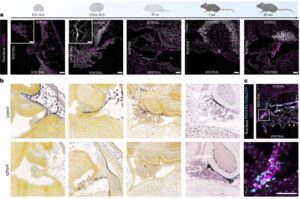
Scientists discover new gatekeeper cell in the brain
Researchers have identified and characterized a previously unknown cellular barrier in the brain, which sheds new light on how the brain is protected from the rest of the body.

Researchers have identified and characterized a previously unknown cellular barrier in the brain, which sheds new light on how the brain is protected from the rest of the body.

While other drugs clear existing plaques, levetiracetam prevents production of toxic amyloid beta peptides

BURLINGTON, N.C., Feb. 11, 2026 /PRNewswire/ — Labcorp (NYSE: LH), a global leader of innovative and comprehensive laboratory services, announced today the nationwide availability of the Elecsys® pTau-181 test, the first and only blood test cleared by the U.S. Food and Drug Administration (FDA) to aid in the initial assessment of Alzheimer’s disease in the primary care setting. This launch further expands Labcorp’s comprehensive portfolio of Alzheimer’s disease blood tests, offering clinicians solutions across both primary and specialty care settings.
A wearable biosensor developed by Washington State University researchers could improve wireless glucose monitoring for people with diabetes, making it more cost-effective, accurate, and less invasive than current models.
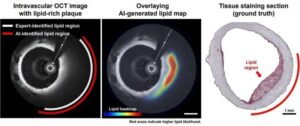
Researchers have developed a new artificial intelligence-based approach for detecting fatty deposits inside coronary arteries using optical coherence tomography (OCT) images.
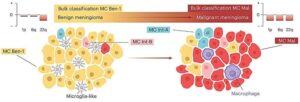
“By using a simple and inexpensive technique that pathologists already use every day, it is now possible to make a better risk assessment, even in countries where advanced technologies are not available.”

New research from McMaster University suggests that disruptions in a person’s sleep and daily activity routine, as detected through a simple wrist-worn device, can signal when there is increased risk of relapsing into major depression.
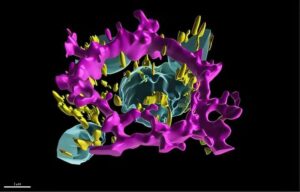
National Institutes of Health (NIH) researchers have developed a digital replica of crucial eye cells, providing a new tool for studying how the cells organize themselves when they are healthy and affected by diseases.

Researchers at the University of Arkansas have developed an artificial-intelligence diagnostic tool to detect masked hypertension.
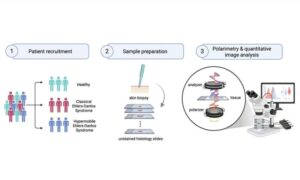
In a recent study by researchers in Toronto (Canada), scientists tested an optical method called Mueller matrix polarimetry to address a diagnostic gap.