MedTech News
.................... by Andrew Celentano
AI stethoscope can help spot ‘silent epidemic’ of heart valve disease earlier than GPs, study suggests
Artificial intelligence could help doctors detect serious heart valve disease years earlier, potentially saving thousands of lives, a new study suggests.

3D-printed brain models could improve medical research and training
University of Missouri researchers are developing new ways to better simulate the complex nature of human brain tissue.
Gamma-synced brain stimulation can nudge people to behave less selfishly
Stimulating two brain areas, nudging them to collectively fire in the same way, increases a person’s ability to behave altruistically.

Scientists create ‘smart underwear’ to measure human flatulence
Scientists at the University of Maryland have created Smart Underwear, the first wearable device designed to measure human flatulence.
TransMedics Receives Full and Unconditional FDA IDE Approval for Next-Generation OCS Heart ENHANCE Trial
ANDOVER, Mass., Feb. 9, 2026 /PRNewswire/ — TransMedics Group, Inc. (“TransMedics”) (Nasdaq: TMDX), a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart, and liver failure, today announced that the U.S. Food and Drug Administration (FDA) has granted full approval of its Investigational Device Exemption (IDE) for the Next-Generation OCS ENHANCE Heart trial.

AI model can accelerate antibody drug production
Researchers detail a machine learning model that dramatically accelerates the manufacturing timeline of monoclonal antibodies.
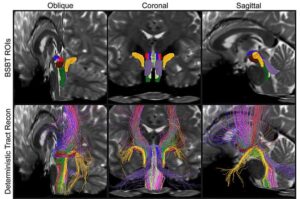
AI algorithm enables tracking of brainstem’s vital white matter pathways
Research reveals distinct patterns of structural changes in patients with Parkinson’s disease, multiple sclerosis, and traumatic brain injury.

Simple patch can make medications safer and more effective
Researchers working alongside Australian diagnostics company Nutromics developed a minimally invasive patch that tracks the antibiotic in patients every five minutes.
