MedTech News
.................... by Andrew Celentano

Neurophet Secures FDA 510(k) Clearance for “Neurophet AQUA AD Plus,” Marking Its Third U.S. Regulatory Milestone
SEOUL, South Korea, Feb. 6, 2026 /PRNewswire/ — Neurophet (Co-CEOs Jake Junkil Been and Donghyeon Kim), an artificial intelligence (AI) solution company for brain disorders diagnosis and treatment, announced today that its software solution, Neurophet AQUA AD Plus, a comprehensive neuroimaging analysis solution for clinical evaluation related to Alzheimer’s disease, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
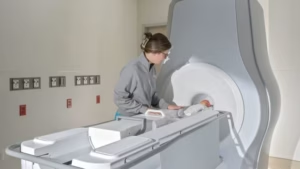
FDA grants clearance for Eyas Medical Imaging’s neonatal MRI system
Eyas Medical Imaging is currently scaling up operations with plans to commercialise the system in the US later this year.
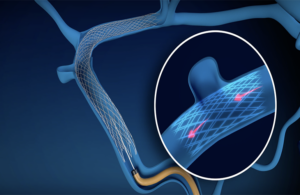
Sonorous Neurovascular earns FDA breakthrough mark for novel stent
Sonorous Neurovascular received FDA breakthrough device designation for its BosSTENT device intended to treat pulsatile tinnitus, the company announced on Thursday.

Tiny ‘mini-me’ organs grown from children’s cells are transforming cystic fibrosis care
More than 2,000 known CFTR mutations have been identified worldwide. The type of mutation a patient carries can alter everything from how severe their symptoms are to what drugs will work for them.
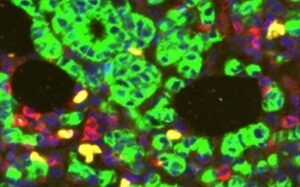
Why some breast cancers evade treatment: Protein secreted by T cells may explain resistant tumors
A study led by researchers at UT Southwestern, published in the Journal of Clinical Investigation, suggests that a protein secreted by immune cells within these tumors causes them to grow even in the absence of estrogen.
New tool spots early signs of infection after breast cancer reconstruction
Researchers at Washington University School of Medicine in St. Louis have developed a new tool to detect reconstruction-related infections early, before they cause symptoms.
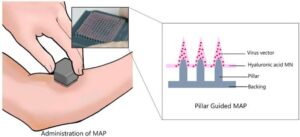
A 3D-printed delivery system enhances vaccine delivery via microneedle array patch
Researchers from the Institute of Industrial Science, The University of Tokyo have used 3D-printing technology to improve the viral titer of microneedle array patches, resulting in effective immunogenicity and protection against infection in mice.
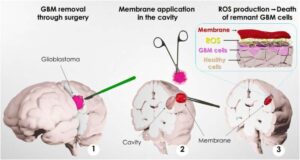
Experimental bioadhesive patch sticks to wet brain tissue and wipes out most glioblastoma cells
A research team led by Professor Víctor Yuste, from the Department of Biochemistry and Molecular Biology and the Institut de Neurociències de la UAB (INc-UAB), has designed and tested several bioadhesive patches that could be placed at the site where the tumor is removed during surgery, targeting any remaining cancer cells.
