MedTech News
.................... by Andrew Celentano

Terahertz microscope reveals the motion of superconducting electrons
For the first time, the new scope allowed physicists to observe terahertz “jiggles” in a superconducting fluid.

TSC Life wins FDA clearance for pediatric use of Fluido system
TSC Life announced that it received FDA clearance for its Fluido Compact fluid warming system for pediatric use.
Testing menstrual blood for HPV could be ‘robust alternative’ to cervical screening
Testing menstrual blood for human papillomavirus (HPV) could be a “robust alternative or replacement” for current cervical cancer screening by a clinician.

Spray away infections: New device delivers antibiotics via mist, alleviating risks of side effects
A University of Missouri researcher has unveiled a safer, smarter way to fight drug-resistant infections.
Self-regulating living implant could end daily insulin injections
A pioneering study marks a major step toward eliminating the need for daily insulin injections for people with diabetes.
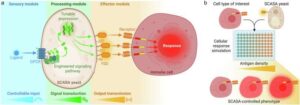
Yeast cells can be used for rapid testing of cancer immunotherapy
An international research team with strong participation from DTU has developed a new biotechnological platform that makes it possible to test and understand advanced cancer treatments much faster and cheaper than before
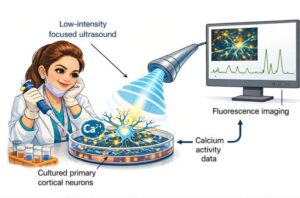
A new way to communicate with neurons using focused ultrasound stimulation
I still vividly remember the first time we observed neurons responding not to audible sound, but to concentrated, precisely calibrated ultrasonic pulses.

Unexpected partial recovery of natural vision observed after intracortical microstimulation in a blind patient
A patient with complete blindness caused by irreversible optic nerve damage partially recovered natural vision after participating in a clinical trial of electrical stimulation of the visual cortex conducted by researchers.
